Probing the nuclear import signal and nuclear transport molecular determinants of PRV ICP22
- PMID: 26816613
- PMCID: PMC4727382
- DOI: 10.1186/s13578-016-0069-7
Probing the nuclear import signal and nuclear transport molecular determinants of PRV ICP22
Abstract
Background: Herpes simplex virus 1 (HSV-1) ICP22 is a multifunctional protein and important for HSV-1 replication. Pseudorabies virus (PRV) ICP22 (P-ICP22) is a homologue of HSV-1 ICP22 and is reported to be able to selectively modify the transcription of different kinetic classes of PRV genes, however, the subcellular localization, localization signal and molecular determinants for its transport to execute this function is less well understood.
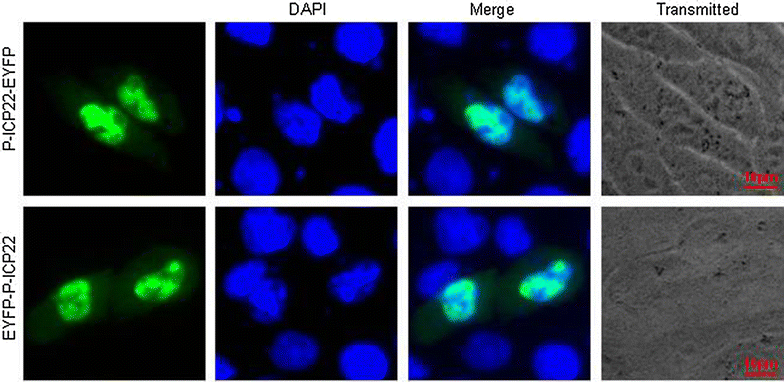
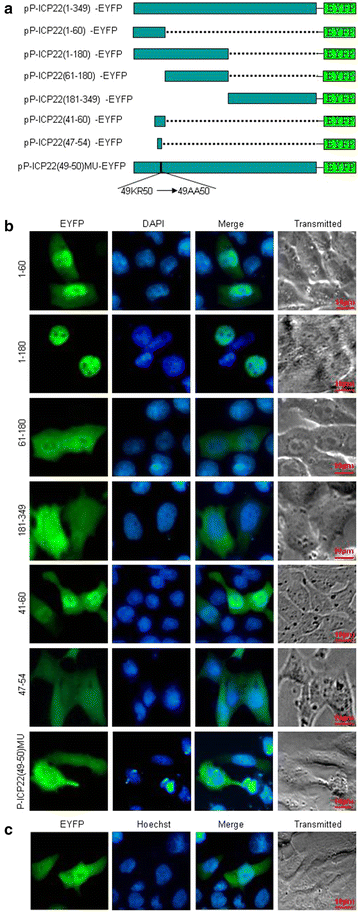
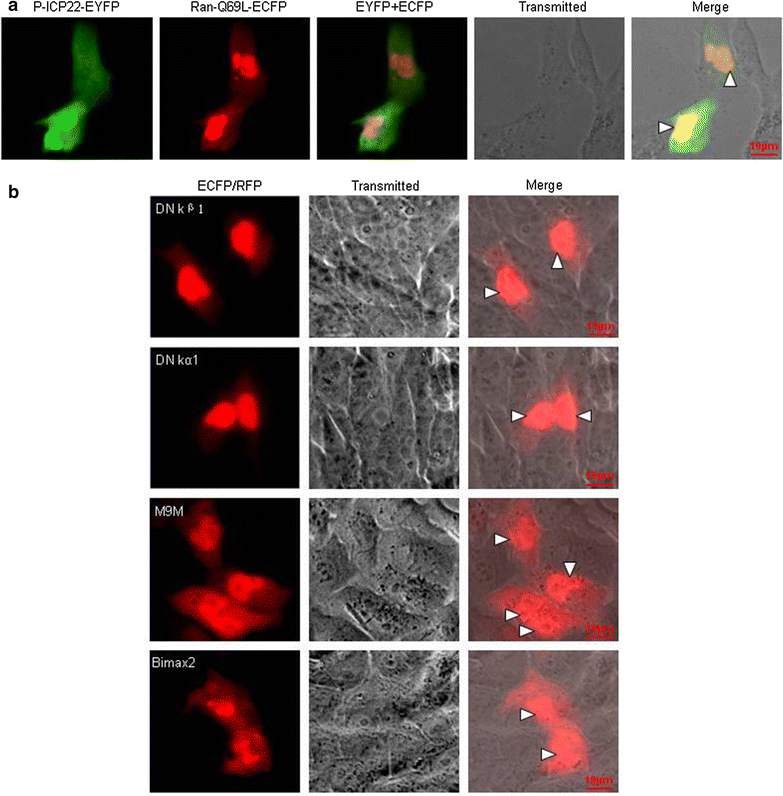
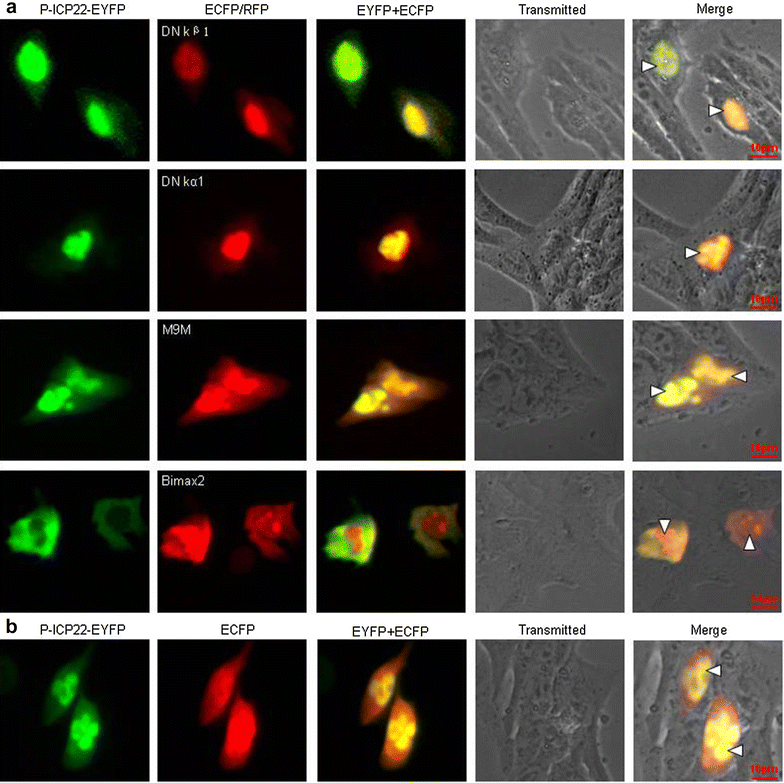
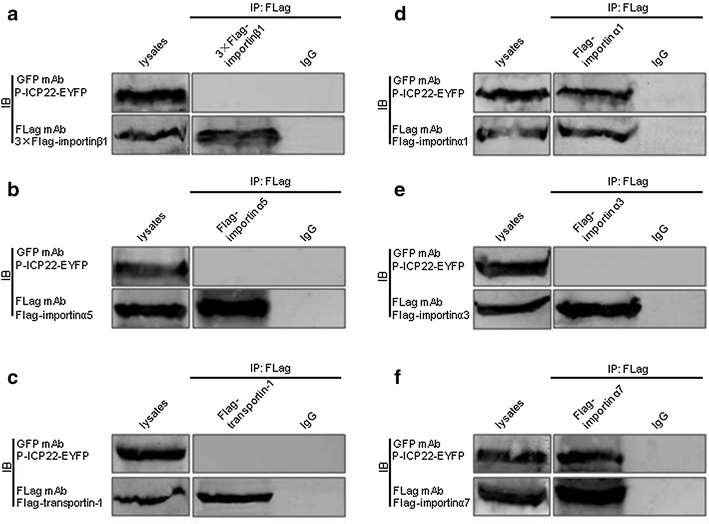
Results: In this study, by utilizing live cells fluorescent microscopy, P-ICP22 fused to enhanced yellow fluorescent protein (EYFP) gene was transient expressed in live cells and shown to exhibit a predominantly nucleus localization in the absence of other viral proteins. By transfection of a series of P-ICP22 deletion mutants fused to EYFP, a bona fide nuclear localization signal (NLS) and its key amino acids (aa) of P-ICP22 was, for the first time, determined and mapped to aa 41-60 (PASTPTPPKRGRYVVEHPEY) and aa 49-50 (KR), respectively. Besides, the P-ICP22 was demonstrated to be targeted to the nucleus via Ran-, importin α1-, and α7-mediated pathway.
Conclusions: Our findings reported herein disclose the NLS and molecular mechanism for nuclear transport of P-ICP22, these results will uncover new avenues for depicting the biological roles of P-ICP22 during PRV infection.
Keywords: Importin; Nuclear localization signal (NLS); Nuclear transport; PRV ICP22; Ran-GTP.
Figures
Similar articles
-
Identification of molecular determinants for the nuclear import of pseudorabies virus UL31.Arch Biochem Biophys. 2015 Dec 1;587:12-7. doi: 10.1016/j.abb.2015.09.024. Epub 2015 Oct 9. Arch Biochem Biophys. 2015. PMID: 26450651
-
Characterization of the nuclear import and export signals of pseudorabies virus UL31.Arch Virol. 2015 Oct;160(10):2591-4. doi: 10.1007/s00705-015-2527-7. Epub 2015 Jul 21. Arch Virol. 2015. PMID: 26195191
-
Identification of the molecular determinants for nuclear import of PRV EP0.Biol Chem. 2019 Sep 25;400(10):1385-1394. doi: 10.1515/hsz-2019-0201. Biol Chem. 2019. PMID: 31120855
-
Identification of two nuclear import signals in the alpha-gene product ICP22 of herpes simplex virus 1.Virology. 2002 Apr 10;295(2):360-70. doi: 10.1006/viro.2002.1384. Virology. 2002. PMID: 12033795
-
Nucleocytoplasmic protein transport and recycling of Ran.Cell Struct Funct. 1999 Dec;24(6):425-33. doi: 10.1247/csf.24.425. Cell Struct Funct. 1999. PMID: 10698256 Review.
Cited by
-
Characterization of the subcellular localization of Epstein-Barr virus encoded proteins in live cells.Oncotarget. 2017 Jul 25;8(41):70006-70034. doi: 10.18632/oncotarget.19549. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050259 Free PMC article.
-
ICP22/IE63 Mediated Transcriptional Regulation and Immune Evasion: Two Important Survival Strategies for Alphaherpesviruses.Front Immunol. 2021 Dec 2;12:743466. doi: 10.3389/fimmu.2021.743466. eCollection 2021. Front Immunol. 2021. PMID: 34925320 Free PMC article. Review.
-
"Non-Essential" Proteins of HSV-1 with Essential Roles In Vivo: A Comprehensive Review.Viruses. 2020 Dec 23;13(1):17. doi: 10.3390/v13010017. Viruses. 2020. PMID: 33374862 Free PMC article. Review.
-
The Molecular Mechanism of Herpes Simplex Virus 1 UL31 in Antagonizing the Activity of IFN-β.Microbiol Spectr. 2022 Feb 23;10(1):e0188321. doi: 10.1128/spectrum.01883-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196784 Free PMC article.
-
Molecular anatomy of the subcellular localization and nuclear import mechanism of herpes simplex virus 1 UL6.Aging (Albany NY). 2020 Apr 1;12(7):5751-5763. doi: 10.18632/aging.102965. Epub 2020 Apr 1. Aging (Albany NY). 2020. PMID: 32235005 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

