Nanofiber-microsphere (nano-micro) matrices for bone regenerative engineering: a convergence approach toward matrix design
- PMID: 26816620
- PMCID: PMC4669008
- DOI: 10.1093/rb/rbu002
Nanofiber-microsphere (nano-micro) matrices for bone regenerative engineering: a convergence approach toward matrix design
Abstract
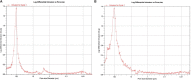
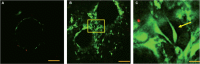
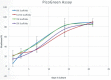
Bone is an essential organ for health and quality of life. Due to current shortfalls in therapy for bone tissue engineering, scientists have sought the application of synthetic materials as bone graft substitutes. As a composite organic/inorganic material with significant extra cellular matrix (ECM), one way to improve bone graft substitutes may be to engineer a synthetic matrix that is influenced by the physical appearance of natural ECM networks. In this work, the authors evaluate composite, hybrid scaffolds for bone tissue engineering based on composite ceramic/polymer microsphere scaffolds with synthetic ECM-mimetic networks in their pore spaces. Using thermally induced phase separation, nanoscale fibers were deposited in the pore spaces of structurally sound microsphere-based scaffold with a density proportionate to the initial polymer concentration. Porosimetry and mechanical testing indicated no significant changes in overall pore characteristics or mechanical integrity as a result of the fiber deposition process. These scaffolds displayed adequate mechanical integrity on the scale of human trabecular bone and supported the adhesion and proliferation of cultured mouse calvarial osteoblasts. Drawing from natural cues, these scaffolds may represent a new avenue forward for advanced bone tissue engineering scaffolds.
Keywords: bioceramics; bone; medical device; nanobiomaterials.
Figures




Similar articles
-
Cellulose and collagen derived micro-nano structured scaffolds for bone tissue engineering.J Biomed Nanotechnol. 2013 Apr;9(4):719-31. doi: 10.1166/jbn.2013.1574. J Biomed Nanotechnol. 2013. PMID: 23621034
-
Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair.Biomaterials. 2003 Feb;24(4):597-609. doi: 10.1016/s0142-9612(02)00374-5. Biomaterials. 2003. PMID: 12437954
-
Tissue engineered microsphere-based matrices for bone repair: design and evaluation.Biomaterials. 2002 Jan;23(2):551-9. doi: 10.1016/s0142-9612(01)00137-5. Biomaterials. 2002. PMID: 11761175
-
Enhanced osteoblast adhesion on polymeric nano-scaffolds for bone tissue engineering.J Biomed Nanotechnol. 2011 Apr;7(2):238-44. doi: 10.1166/jbn.2011.1283. J Biomed Nanotechnol. 2011. PMID: 21702361 Review.
-
Taking cues from the extracellular matrix to design bone-mimetic regenerative scaffolds.Matrix Biol. 2016 May-Jul;52-54:397-412. doi: 10.1016/j.matbio.2016.02.011. Epub 2016 Mar 2. Matrix Biol. 2016. PMID: 26940231 Free PMC article. Review.
Cited by
-
Development of an antimicrobial peptide-loaded mineralized collagen bone scaffold for infective bone defect repair.Regen Biomater. 2020 Apr 24;7(5):515-525. doi: 10.1093/rb/rbaa015. eCollection 2020 Oct. Regen Biomater. 2020. PMID: 33149940 Free PMC article.
-
Magnesium phosphate functionalized graphene oxide and PLGA composite matrices with enhanced mechanical and osteogenic properties for bone regeneration.Regen Biomater. 2025 Jul 26;12:rbaf074. doi: 10.1093/rb/rbaf074. eCollection 2025. Regen Biomater. 2025. PMID: 40837704 Free PMC article.
-
In vivo inducing collagen regeneration of biodegradable polymer microspheres.Regen Biomater. 2021 Jul 15;8(5):rbab042. doi: 10.1093/rb/rbab042. eCollection 2021 Oct. Regen Biomater. 2021. PMID: 34408912 Free PMC article.
-
A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications.Polymers (Basel). 2020 Sep 8;12(9):2043. doi: 10.3390/polym12092043. Polymers (Basel). 2020. PMID: 32911705 Free PMC article. Review.
-
Nanofiber/Microsphere Hybrid Matrices In Vivo for Bone Regenerative Engineering: A Preliminary Report.Regen Eng Transl Med. 2018 Sep;4(3):133-141. doi: 10.1007/s40883-018-0055-1. Epub 2018 Jun 14. Regen Eng Transl Med. 2018. PMID: 30687776 Free PMC article.
References
-
- Borden M, Attawia M, Khan Y, et al. Tissue engineered microsphere-based matrices for bone repair: design and evaluation. Biomaterials 2002;23:551–9. - PubMed
-
- Calori GM, Mazza E, Colombo M, et al. The use of bone-graft substitutes in large bone defects: any specific needs? Injury 2011;42(Suppl. 2):S56–63. - PubMed
-
- Kolk A, Handschel J, Drescher W, et al. Current trends and future perspectives of bone substitute materials—from space holders to innovative biomaterials. J Craniomaxillofac Surg 2012;40:706–18. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

