Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds
- PMID: 26816627
- PMCID: PMC4669006
- DOI: 10.1093/rb/rbu009
Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds
Abstract
Heart valve and blood vessel replacement using artificial prostheses is an effective strategy for the treatment of cardiovascular disease at terminal stage. Natural extracellular matrix (ECM)-derived materials (decellularized allogeneic or xenogenic tissues) have received extensive attention as the cardiovascular scaffold. However, the bioprosthetic grafts usually far less durable and undergo calcification and progressive structural deterioration. Glutaraldehyde (GA) is a commonly used crosslinking agent for improving biocompatibility and durability of the natural scaffold materials. However, the nature ECM and GA-crosslinked materials may result in calcification and eventually lead to the transplant failure. Therefore, studies have been conducted to explore new crosslinking agents. In this review, we mainly focused on research progress of ECM-derived cardiovascular scaffolds and their crosslinking strategies.
Keywords: calcification; cross-linking agent; extracellular matrix; scaffold; tissue engineering.
Figures

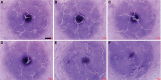






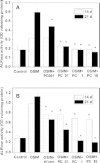

References
-
- Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol 2003;41:893–904. - PubMed
-
- Julie RF, Boris AN, Joseph PV. Tissue engineering: a 21st century solution to surgical reconstruction. Ann Thorac Surg 2001;72:577–91. - PubMed
-
- Cannegieter SC, Rosendaal FR, Briet E. Thrombembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994;89:635–41. - PubMed
-
- Ralf S, Simon PH, Jason SS, et al. Tissue engineering of heart valves: in vitro experiences. Ann Thorac Surg 2000;70:140–4. - PubMed
-
- Kirklin JK, Smith D, Novick W, et al. Long-term function of cryopreserved aortic homografts. J Thorac Cardiovasc Surg 1993;106:154–66. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

