Novel non-AR therapeutic targets in castrate resistant prostate cancer
- PMID: 26816739
- PMCID: PMC4708177
- DOI: 10.3978/j.issn.2223-4683.2013.09.09
Novel non-AR therapeutic targets in castrate resistant prostate cancer
Abstract
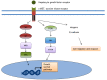
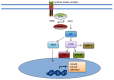
Castrate resistant prostate cancer (CRPC) remains a disease with significant morbidity and mortality. The recent approval of abiraterone and enzalutamide highlight the improvements which can be made targeting the androgen receptor (AR) axis. Nonetheless, resistance inevitably develops and there is continued interest in targeting alternate pathways which cause disease resistance and progression. Here, we review non-AR targets in CRPC, with an emphasis on novel agents now in development. This includes therapeutics which target the tumour microenvironment, the bone metastatic environment, microtubules, cellular energetics, angiogenesis, the stress response, survival proteins, intracellular signal transduction, DNA damage repair and dendritic cells. Understanding the hallmarks of prostate cancer resistance in CRPC has led to the identification and development of these new targets. We review the molecular rationale, as well at the clinical experience for each of these different classes of agents which are in clinical development.
Keywords: Androgen receptor (AR); abiraterone; castrate resistant prostate cancer (CRPC); non-AR therapeutic targets.
Conflict of interest statement
Figures
References
-
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;168:9-12. - PubMed
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
