Treatment of painful bladder syndrome/interstitial cystitis with botulinum toxin A: why isn't it effective in all patients?
- PMID: 26816853
- PMCID: PMC4708559
- DOI: 10.3978/j.issn.2223-4683.2015.10.02
Treatment of painful bladder syndrome/interstitial cystitis with botulinum toxin A: why isn't it effective in all patients?
Abstract
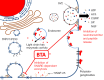
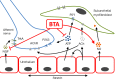
Botulinum toxin A (BTA) is currently used to treat a variety of painful disorders, including painful bladder syndrome/interstitial cystitis (PBS/IC). However, BTA is not consistently effective in all patients. This may be due to the disparity of causes of pain, but this may also relate to the processes by which BTA exerts anti-nociceptive effects. This review discusses mechanisms by which BTA may inhibit pain and studies of the use of BTA in PSB/IC patients. It is doubtful that any single treatment will effectively control pain in PBS/IC patients, and it is highly probable that multiple strategies will be required, both within individual patients and across the population of PBS/IC patients. The purpose of this review is to discuss those mechanisms by which BTA acts, with the intent that alternative strategies exploiting these mechanism, or work through alternative pathways, can be identified to more effectively treat pain in PBS/IC patients in the future.
Keywords: Painful bladder syndrome/interstitial cystitis (PBS/IC); botulinum toxin A (BTA); treatment.
Conflict of interest statement
Figures
References
-
- Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139:919-22. - PubMed
-
- Leippold T, Reitz A, Schurch B. Botulinum toxin as a new therapy option for voiding disorders: current state of the art. Eur Urol 2003;44:165-74. - PubMed
-
- Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000;38:245-58. - PubMed
-
- Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol 2001;8 Suppl 5:21-9. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
