Efficacy of Herpes Simplex Virus Vector Encoding the Human Preproenkephalin Gene for Treatment of Facial Pain in Mice
- PMID: 26817032
- PMCID: PMC4874570
- DOI: 10.11607/ofph.1512
Efficacy of Herpes Simplex Virus Vector Encoding the Human Preproenkephalin Gene for Treatment of Facial Pain in Mice
Abstract
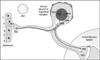
Aims: To determine whether herpes simplex virus-based vectors can efficiently transduce mouse trigeminal ganglion (TG) neurons and attenuate preexisting nerve injury-induced whisker pad mechanical hypersensitivity in a trigeminal inflammatory compression (TIC) neuropathic pain model.
Methods: Tissue transduction efficiencies of replication-conditional and replication-defective vectors to mouse whisker pads after topical administration and subcutaneous injection were assessed using quantitative real-time PCR (qPCR). Tissue tropism and transgene expression were assessed using qPCR and reverse-transcriptase qPCR following topical application of the vectors. Whisker pad mechanical sensitivities of TIC-injured mice were determined using graduated von Frey fibers before and after application of human preproenkephalin expressing replication-conditional vector (KHPE). Data were analyzed using one-way analysis of variance (ANOVA) and post hoc tests.
Results: Transduction of target TGs was 8- to 50-fold greater after topical application than subcutaneous injection and ≥ 100-fold greater for replication-conditional than replication-defective vectors. Mean KHPE loads remained constant in TGs (4.5-9.8 × 10(4) copies/TG) over 3 weeks but were below quantifiable levels (10 copies/tissue) within 2 weeks of application in other nontarget cephalic tissues examined. Transgene expression in TGs was maximal during 2 weeks after topical application (100-200 cDNA copies/mL) and was below quantifiable levels (1 cDNA copy/mL) in all nontarget tissues. Topical KHPE administration reduced TIC-related mechanical hypersensitivity on whisker pads 4-fold (P < .05) for at least 1 week.
Conclusion: Topically administered KHPE produced a significant antinociceptive effect in the TIC mouse model of chronic facial neuropathic pain. This is the first report in which a gene therapeutic approach reduced trigeminal pain-related behaviors in an established pain state in mice.
Conflict of interest statement
The authors report no conflicts of interest related to this study.
Figures




Similar articles
-
Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons.Mol Ther. 2005 Apr;11(4):608-16. doi: 10.1016/j.ymthe.2004.12.011. Mol Ther. 2005. PMID: 15771963
-
Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin.Hum Gene Ther. 2009 Jan;20(1):63-71. doi: 10.1089/hum.2008.094. Hum Gene Ther. 2009. PMID: 20377371 Free PMC article.
-
Nasal application of HSV encoding human preproenkephalin blocks craniofacial pain in a rat model of traumatic brain injury.Gene Ther. 2017 Aug;24(8):482-486. doi: 10.1038/gt.2017.55. Epub 2017 Jul 20. Gene Ther. 2017. PMID: 28682314
-
Gene delivery using herpes simplex virus vectors.DNA Cell Biol. 2002 Dec;21(12):915-36. doi: 10.1089/104454902762053864. DNA Cell Biol. 2002. PMID: 12573050 Review.
-
Herpes virus-based recombinant herpes vectors: gene therapy for pain and molecular tool for pain science.Gene Ther. 2009 Apr;16(4):502-8. doi: 10.1038/gt.2009.25. Epub 2009 Feb 19. Gene Ther. 2009. PMID: 19225546 Review.
Cited by
-
Diabetic neuropathic pain induced by streptozotocin alters the expression profile of non-coding RNAs in the spinal cord of mice as determined by sequencing analysis.Exp Ther Med. 2021 Jul;22(1):775. doi: 10.3892/etm.2021.10207. Epub 2021 May 18. Exp Ther Med. 2021. PMID: 34055074 Free PMC article.
-
Overexpression of µ-Opioid Receptors in Peripheral Afferents, but Not in Combination with Enkephalin, Decreases Neuropathic Pain Behavior and Enhances Opioid Analgesia in Mouse.Anesthesiology. 2018 May;128(5):967-983. doi: 10.1097/ALN.0000000000002063. Anesthesiology. 2018. PMID: 29334500 Free PMC article.
-
Epigenetic modifications associated to diabetic peripheral neuropathic pain (Review).Mol Med Rep. 2025 Jan;31(1):28. doi: 10.3892/mmr.2024.13394. Epub 2024 Nov 14. Mol Med Rep. 2025. PMID: 39540354 Free PMC article. Review.
-
Gene Therapy: A Paradigm Shift in Dentistry.Genes (Basel). 2016 Nov 10;7(11):98. doi: 10.3390/genes7110098. Genes (Basel). 2016. PMID: 27834914 Free PMC article. Review.
-
Histone deacetylase inhibitors prevent persistent hypersensitivity in an orofacial neuropathic pain model.Mol Pain. 2018 Jan-Dec;14:1744806918796763. doi: 10.1177/1744806918796763. Mol Pain. 2018. PMID: 30178698 Free PMC article.
References
-
- Glorioso JC, Mata M, Fink DJ. Therapeutic gene transfer to the nervous system using viral vectors. J Neurovirol. 2003;9:165–172. - PubMed
-
- Kumar S, Ruchi R, James SR, Chidiac EJ. Gene therapy for chronic neuropathic pain: How does it work and where do we stand today? Pain Med. 2011;12:808–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

