Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity
- PMID: 26817498
- PMCID: PMC4966846
- DOI: 10.1080/19420862.2015.1136761
Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity
Abstract
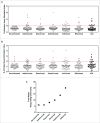
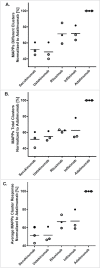
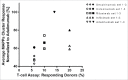
Secukinumab is a human monoclonal antibody that selectively targets interleukin-17A and has been demonstrated to be highly efficacious in the treatment of moderate to severe plaque psoriasis, starting at early time points, with a sustained effect and a favorable safety profile. Biotherapeutics--including monoclonal antibodies (mAbs)--can be immunogenic, leading to formation of anti-drug antibodies (ADAs) that can result in unwanted effects, including hypersensitivity reactions or compromised therapeutic efficacy. To gain insight into possible explanations for the clinically observed low immunogenicity of secukinumab, we evaluated its immunogenicity potential by applying 2 different in vitro assays: T-cell activation and major histocompatibility complex-associated peptide proteomics (MAPPs). For both assays, monocyte-derived dendritic cells (DCs) from healthy donors were exposed in vitro to biotherapeutic proteins. DCs naturally process proteins and present the derived peptides in the context of human leukocyte antigen (HLA)-class II. HLA-DR-associated biotherapeutic-derived peptides, representing potential T-cell epitopes, were identified in the MAPPs assay. In the T-cell assay, autologous CD4(+) T cells were co-cultured with secukinumab-exposed DCs and T-cell activation was measured by proliferation and interleukin-2 secretion. In the MAPPs analysis and T-cell activation assays, secukinumab consistently showed relatively low numbers of potential T-cell epitopes and low T-cell response rates, respectively, comparable to other biotherapeutics with known low clinical immunogenicity. In contrast, biotherapeutics with elevated clinical immunogenicity rates showed increased numbers of potential T-cell epitopes and increased T-cell response rates in T-cell activation assays, indicating an approximate correlation between in vitro assay results and clinical immunogenicity incidence.
Keywords: AIN457; Anti-drug antibodies; IL-17A; T-cell assay; antigen presentation assay; dendritic cell; immunogenicity prediction; major histocompatibility complex-associated peptide proteomics; psoriasis; secukinumab.
Figures


Similar articles
-
T cell epitope mapping of secukinumab and ixekizumab in healthy donors.MAbs. 2020 Jan-Dec;12(1):1707418. doi: 10.1080/19420862.2019.1707418. MAbs. 2020. PMID: 31924123 Free PMC article.
-
Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis.Br J Dermatol. 2017 Mar;176(3):752-758. doi: 10.1111/bjd.14965. Epub 2016 Nov 22. Br J Dermatol. 2017. PMID: 27518376
-
Internalization of therapeutic antibodies into dendritic cells as a risk factor for immunogenicity.Front Immunol. 2024 Aug 28;15:1406643. doi: 10.3389/fimmu.2024.1406643. eCollection 2024. Front Immunol. 2024. PMID: 39263220 Free PMC article.
-
Applying MAPPs Assays to Assess Drug Immunogenicity.Front Immunol. 2020 Apr 21;11:698. doi: 10.3389/fimmu.2020.00698. eCollection 2020. Front Immunol. 2020. PMID: 32373128 Free PMC article. Review.
-
Secukinumab (AIN457) for the treatment of psoriasis.Expert Rev Clin Immunol. 2015;11(11):1177-88. doi: 10.1586/1744666X.2015.1095092. Epub 2015 Oct 1. Expert Rev Clin Immunol. 2015. PMID: 26428036 Review.
Cited by
-
Treatment challenges in the management of moderate-to-severe plaque psoriasis - role of secukinumab.Clin Cosmet Investig Dermatol. 2016 Oct 11;9:347-355. doi: 10.2147/CCID.S81160. eCollection 2016. Clin Cosmet Investig Dermatol. 2016. PMID: 27785085 Free PMC article. Review.
-
In silico methods for immunogenicity risk assessment and human homology screening for therapeutic antibodies.MAbs. 2024 Jan-Dec;16(1):2333729. doi: 10.1080/19420862.2024.2333729. Epub 2024 Mar 27. MAbs. 2024. PMID: 38536724 Free PMC article.
-
Immunogenicity to biological drugs in psoriasis and psoriatic arthritis.Clinics (Sao Paulo). 2021 Oct 1;76:e3015. doi: 10.6061/clinics/2021/e3015. eCollection 2021. Clinics (Sao Paulo). 2021. PMID: 34614113 Free PMC article.
-
Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis.Hum Vaccin Immunother. 2017 Oct 3;13(10):2247-2259. doi: 10.1080/21645515.2017.1356498. Hum Vaccin Immunother. 2017. PMID: 28825875 Free PMC article. Review.
-
Improved prediction of HLA antigen presentation hotspots: Applications for immunogenicity risk assessment of therapeutic proteins.Immunology. 2021 Feb;162(2):208-219. doi: 10.1111/imm.13274. Epub 2020 Oct 19. Immunology. 2021. PMID: 33010039 Free PMC article.
References
-
- Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, Russell CB. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013; 133:17-26; PMID:22673731; http://dx.doi.org/10.1038/jid.2012.194 - DOI - PMC - PubMed
-
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008; 128:1207-11; PMID:18200064; http://dx.doi.org/10.1038/sj.jid.5701213 - DOI - PubMed
-
- Johansen C, Usher P, Kjellerup RB, Lundsgaard D, Iverson L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol 2009; 160:319-24; PMID:19016708; http://dx.doi.org/10.1111/j.1365-2133.2008.08902.x - DOI - PubMed
-
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villaneuva EC, Shah P, Kaplan MJ, Bruce AT. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 2011; 187:490-500; PMID:21606249; http://dx.doi.org/10.4049/jimmunol.1100123 - DOI - PMC - PubMed
-
- Res PC, Piskin G, deBoer OJ, van der Loos CM, Teeling P, Bos JD, Teunissen MB. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggest their involvement in the pathogenesis of psoriasis. PLoS One 2010; 5:e14108; PMID:21124836; http://dx.doi.org/10.1371/journal.pone.0014108 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
