Safety and tolerability of azilsartan medoxomil in subjects with essential hypertension: a one-year, phase 3, open-label study
- PMID: 26817604
- PMCID: PMC4819839
- DOI: 10.3109/10641963.2015.1081213
Safety and tolerability of azilsartan medoxomil in subjects with essential hypertension: a one-year, phase 3, open-label study
Abstract
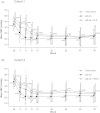
This 56-week phase 3, open-label, treat-to-target study, involving 2 consecutive, non-randomized cohorts, evaluated the safety and tolerability of azilsartan medoxomil (AZL-M) in essential hypertension (mean baseline blood pressure [BP] 152/100 mmHg). All subjects (n = 669) initiated AZL-M 40 mg QD, force-titrated to 80 mg QD at week 4, if tolerated. From week 8, subjects could receive additional medications, starting with chlorthalidone (CLD) 25 mg QD (Cohort 1) or hydrochlorothiazide (HCTZ) 12.5-25 mg QD (Cohort 2), if required, to reach BP targets. Adverse events (AEs) were reported in 75.9% of subjects overall in the two cohorts (73.8% Cohort 1, 78.5% Cohort 2). The most common AEs were dizziness (14.3%), headache (9.9%) and fatigue (7.2%). Transient serum creatinine elevations were more frequent with add-on CLD. Clinic systolic/diastolic BP (observed cases at week 56) decreased by 25.2/18.4 mmHg (Cohort 1) and 24.2/17.9 mmHg (Cohort 2). These results demonstrate that AZL-M is well tolerated over the long term and provides stable BP improvements when used in a treat-to-target BP approach with thiazide-type diuretics.
Keywords: Hypertension; angiotensin receptor blocker; azilsartan; chlorthalidone; diuretic; hydrochlorothiazide.
Figures

References
-
- Edarbi (azilsartan medoxomil) tablets. U.S. prescribing information. Arbor Pharmaceuticals, LLC, Atlanta, GA, USA. Jul 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200796s006lbl.pdf [last accessed 13 Oct 2014]
-
- Edarbi (azilsartan medoxomil) tablets. Summary of product characteristics. Takeda Pharma A/S, Taastrup, Denmark. Oct 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... [last accessed 13 Oct 2014]
-
- Zaiken K, Cheng JW. Azilsartan medoxomil: a new angiotensin receptor blocker. Clin Ther. 2011;33:1577–89. - PubMed
-
- Perry CM. Azilsartan medoxomil: a review of its use in hypertension. Clin Drug Investig. 2012;32:621–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
