Eph-B4 mediates vein graft adaptation by regulation of endothelial nitric oxide synthase
- PMID: 26817610
- PMCID: PMC4958608
- DOI: 10.1016/j.jvs.2015.11.041
Eph-B4 mediates vein graft adaptation by regulation of endothelial nitric oxide synthase
Abstract
Objective: Vein graft adaptation is characterized by loss of expression of the tyrosine kinase receptor Eph-B4, the embryonic determinant of venous identity, without increased expression of its ligand ephrin-B2, the embryonic determinant of arterial identity. Endothelial nitric oxide synthase (eNOS) is an important mediator of vessel remodeling. We hypothesized that the mechanism of action of Eph-B4 during vein graft adaptation might be through regulation of downstream eNOS activity.
Methods: Mouse lung endothelial cells were stimulated with ephrin-B2/Fc, without and with preclustering, without and with the eNOS inhibitor Nω-nitro-l-arginine methyl ester hydrochloride or the Eph-B4 inhibitor NVP-BHG712, and assessed by Western blot and immunofluorescence for eNOS and Eph-B4 phosphorylation. Nitric oxide (NO) production was assessed using an NO-specific chemiluminescence analyzer. Cell migration was assessed using a Transwell assay. Human and mouse vein graft specimens were examined for eNOS activity by Western blot, and vessel remodeling was assessed in vein grafts in wild-type or eNOS knockout mice.
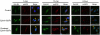
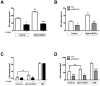
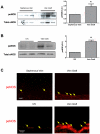
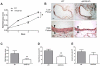
Results: Ephrin-B2/Fc stimulated both Eph-B4 and eNOS phosphorylation in a bimodal temporal distribution (n = 4; P < .05), with preclustered ephrin-B2/Fc causing prolonged peak Eph-B4 and eNOS phosphorylation as well as altered subcellular localization (n = 4; P < .05). Ephrin-B2/Fc increased NO release (n = 3; P < .01) as well as increased endothelial cell migration (n = 6; P < .05) in an eNOS-dependent fashion. Both human and mouse vein grafts showed increased eNOS phosphorylation compared with normal veins (n = 3; P < .05). Vein grafts from eNOS knockout mice showed less dilation and less wall thickening compared with wild-type vein grafts (n = 7; P < .05).
Conclusions: eNOS is a mediator of vein graft adaptation to the arterial environment. Eph-B4 stimulates eNOS phosphorylation in vitro and may mediate vein graft adaptation by regulation of eNOS activity in vivo.
Published by Elsevier Inc.
Figures

Comment in
-
Invited commentary.J Vasc Surg. 2017 Jan;65(1):189. doi: 10.1016/j.jvs.2016.10.072. J Vasc Surg. 2017. PMID: 28010858 No abstract available.
Similar articles
-
Molecular identity of arteries, veins, and lymphatics.J Vasc Surg. 2019 Jan;69(1):253-262. doi: 10.1016/j.jvs.2018.06.195. Epub 2018 Aug 25. J Vasc Surg. 2019. PMID: 30154011 Free PMC article. Review.
-
Ephrin type-B receptor 4 activation reduces neointimal hyperplasia in human saphenous vein in vitro.J Vasc Surg. 2016 Mar;63(3):795-804. doi: 10.1016/j.jvs.2014.09.036. Epub 2014 Oct 24. J Vasc Surg. 2016. PMID: 25446283 Free PMC article.
-
Stimulation of Caveolin-1 Signaling Improves Arteriovenous Fistula Patency.Arterioscler Thromb Vasc Biol. 2019 Apr;39(4):754-764. doi: 10.1161/ATVBAHA.119.312417. Arterioscler Thromb Vasc Biol. 2019. PMID: 30786746 Free PMC article.
-
Role of ephrin B2 in human retinal endothelial cell proliferation and migration.Cell Signal. 2003 Nov;15(11):1011-7. doi: 10.1016/s0898-6568(03)00072-x. Cell Signal. 2003. PMID: 14499344
-
Improving the Outcome of Vein Grafts: Should Vascular Surgeons Turn Veins into Arteries?Ann Vasc Dis. 2017 Mar 24;10(1):8-16. doi: 10.3400/avd.ra.17-00008. Epub 2017 Mar 31. Ann Vasc Dis. 2017. PMID: 29034014 Free PMC article. Review.
Cited by
-
Molecular identity of arteries, veins, and lymphatics.J Vasc Surg. 2019 Jan;69(1):253-262. doi: 10.1016/j.jvs.2018.06.195. Epub 2018 Aug 25. J Vasc Surg. 2019. PMID: 30154011 Free PMC article. Review.
-
Sex Differences in Inflammation During Venous Remodeling of Arteriovenous Fistulae.Front Cardiovasc Med. 2021 Jul 21;8:715114. doi: 10.3389/fcvm.2021.715114. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34368264 Free PMC article. Review.
-
Arterial Venous Differentiation for Vascular Bioengineering.Annu Rev Biomed Eng. 2018 Jun 4;20:431-447. doi: 10.1146/annurev-bioeng-062117-121231. Epub 2018 Apr 11. Annu Rev Biomed Eng. 2018. PMID: 29641908 Free PMC article. Review.
-
Dual Function for Mature Vascular Smooth Muscle Cells During Arteriovenous Fistula Remodeling.J Am Heart Assoc. 2017 Mar 30;6(4):e004891. doi: 10.1161/JAHA.116.004891. J Am Heart Assoc. 2017. PMID: 28360226 Free PMC article.
-
The Pyrazolo[3,4-d]pyrimidine-Based Kinase Inhibitor NVP-BHG712: Effects of Regioisomers on Tumor Growth, Perfusion, and Hypoxia in EphB4-Positive A375 Melanoma Xenografts.Molecules. 2020 Nov 3;25(21):5115. doi: 10.3390/molecules25215115. Molecules. 2020. PMID: 33153234 Free PMC article.
References
-
- Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3(1):104–14. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. - PubMed
-
- Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330(20):1431–8. - PubMed
-
- Wan S, George SJ, Berry C, Baker AH. Vein graft failure: current clinical practice and potential for gene therapeutics. Gene Ther. 2012;19(6):630–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

