c-Myc and viral cofactor Kaposin B co-operate to elicit angiogenesis through modulating miRNome traits of endothelial cells
- PMID: 26817819
- PMCID: PMC4895700
- DOI: 10.1186/s12918-015-0242-3
c-Myc and viral cofactor Kaposin B co-operate to elicit angiogenesis through modulating miRNome traits of endothelial cells
Abstract
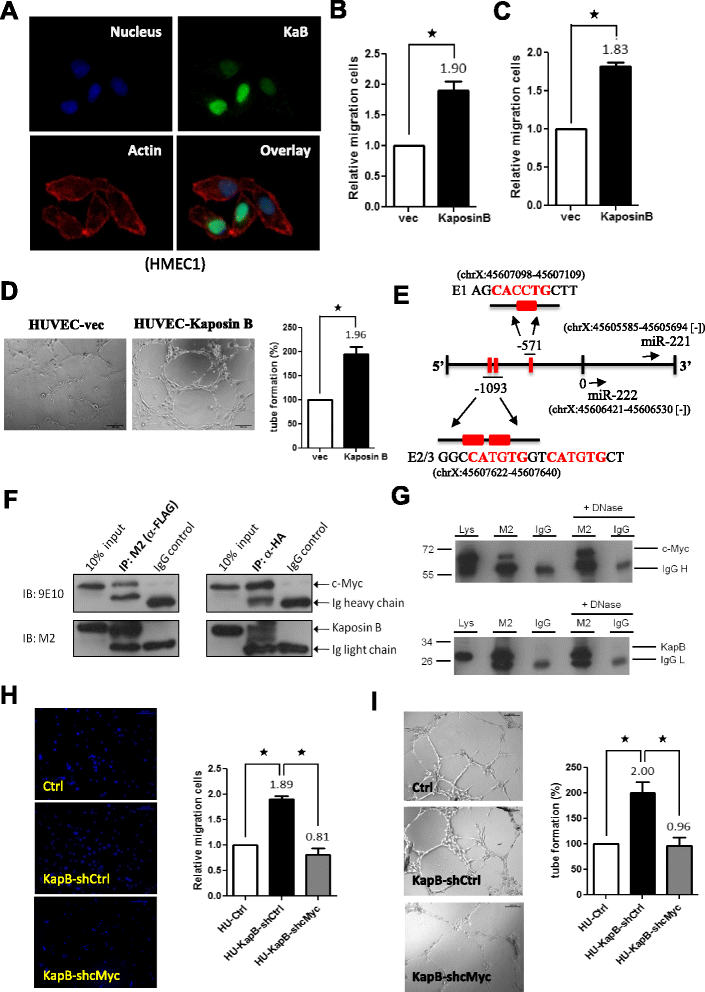
Background: MicroRNAs (miRNAs) have emerged as master regulators of angiogenesis and other cancer-related events. Discovering new angiogenesis-regulating microRNAs (angiomiRs) will eventually help in developing new therapeutic strategies for tumor angiogenesis and cardiovascular diseases. Kaposi's sarcoma (KS), which is induced by the etiological infectious agent KS-associated herpesvirus (KSHV), is a peculiar neoplasm that expresses both blood and lymphatic endothelial markers and possesses extensive neovasculature. Using KSHV and its proteins as baits will be an efficient way to discover new angiomiRs in endothelial cells. Kaposin B is one of the latent viral genes and is expressed in all KSHV tumor cells. Since Kaposin B is a nuclear protein with no DNA-binding domain, it may regulate gene expression by incorporating itself into a transcription complex.
Results: We demonstrated that c-Myc and Kaposin B form a transcription complex and bind to the miR-221/-222 promoter, thereby affecting their expression and anti-angiogenic ability. By small RNA sequencing (smRNA-Seq), we revealed that 72.1% (173/240) of Kaposin B up-regulated and 46.5% (113/243) of Kaposin B down-regulated known miRNAs were regulated by c-Myc. We also found that 77 novel miRNA were up-regulated and 28 novel miRNAs were down-regulated in cells expressing both c-Myc and Kaposin B compared with cells expressing Kaposin B only. The result was confirmed by RNA-IP-seq data.
Conclusions: Our study identifies known and novel c-Myc-regulated microRNAs and reveals that a c-Myc-oriented program is coordinated by Kaposin B in KSHV-infected cells.
Figures




Similar articles
-
Kaposi's sarcoma-associated herpesvirus microRNAs repress breakpoint cluster region protein expression, enhance Rac1 activity, and increase in vitro angiogenesis.J Virol. 2015 Apr;89(8):4249-61. doi: 10.1128/JVI.03687-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631082 Free PMC article.
-
The manipulation of miRNA-gene regulatory networks by KSHV induces endothelial cell motility.Blood. 2011 Sep 8;118(10):2896-905. doi: 10.1182/blood-2011-01-330589. Epub 2011 Jun 28. Blood. 2011. PMID: 21715310
-
MicroRNA-mediated transformation by the Kaposi's sarcoma-associated herpesvirus Kaposin locus.J Virol. 2015 Feb;89(4):2333-41. doi: 10.1128/JVI.03317-14. Epub 2014 Dec 10. J Virol. 2015. PMID: 25505059 Free PMC article.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Posttranscriptional gene regulation in Kaposi's sarcoma-associated herpesvirus.Adv Appl Microbiol. 2009;68:241-61. doi: 10.1016/S0065-2164(09)01206-4. Adv Appl Microbiol. 2009. PMID: 19426857 Review.
Cited by
-
Variation within major internal repeats of KSHV in vivo.Virus Evol. 2023 May 22;9(1):vead034. doi: 10.1093/ve/vead034. eCollection 2023. Virus Evol. 2023. PMID: 37325087 Free PMC article.
-
miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: A systematic review.Mol Ther Nucleic Acids. 2022 Feb 11;27:1191-1224. doi: 10.1016/j.omtn.2022.02.005. eCollection 2022 Mar 8. Mol Ther Nucleic Acids. 2022. PMID: 35282417 Free PMC article. Review.
-
Stealing the Show: KSHV Hijacks Host RNA Regulatory Pathways to Promote Infection.Viruses. 2020 Sep 14;12(9):1024. doi: 10.3390/v12091024. Viruses. 2020. PMID: 32937781 Free PMC article. Review.
-
Metabolic Control by DNA Tumor Virus-Encoded Proteins.Pathogens. 2021 May 6;10(5):560. doi: 10.3390/pathogens10050560. Pathogens. 2021. PMID: 34066504 Free PMC article. Review.
-
The anti-angiogenesis role of FBXW7 in diabetic retinopathy by facilitating the ubiquitination degradation of c-Myc to orchestrate the HDAC2.J Cell Mol Med. 2021 Feb;25(4):2190-2202. doi: 10.1111/jcmm.16204. Epub 2020 Dec 25. J Cell Mol Med. 2021. PMID: 33369138 Free PMC article.
References
-
- Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, et al. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 2: pathogenesis, Castleman’s disease, and pleural effusion lymphoma. Lancet Infect Dis. 2002;2(6):344–352. doi: 10.1016/S1473-3099(02)00288-8. - DOI - PubMed
-
- Hengge UR, Ruzicka T, Tyring SK, Stuschke M, Roggendorf M, Schwartz RA, et al. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2(5):281–292. doi: 10.1016/S1473-3099(02)00263-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

