Ribosome Elongation Stall Directs Gene-specific Translation in the Integrated Stress Response
- PMID: 26817837
- PMCID: PMC4813566
- DOI: 10.1074/jbc.M115.705640
Ribosome Elongation Stall Directs Gene-specific Translation in the Integrated Stress Response
Abstract
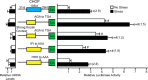
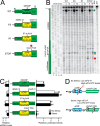
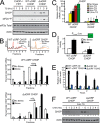
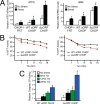
Upon exposure to environmental stress, phosphorylation of the α subunit of eIF2 (eIF2α-P) represses global protein synthesis, coincident with preferential translation of gene transcripts that mitigate stress damage or alternatively trigger apoptosis. Because there are multiple mammalian eIF2 kinases, each responding to different stress arrangements, this translational control scheme is referred to as the integrated stress response (ISR). Included among the preferentially translated mRNAs induced by eIF2α-P is that encoding the transcription factor CHOP (DDIT3/GADD153). Enhanced levels of CHOP promote cell death when ISR signaling is insufficient to restore cell homeostasis. Preferential translation of CHOP mRNA occurs by a mechanism involving ribosome bypass of an inhibitory upstream ORF (uORF) situated in the 5'-leader of the CHOP mRNA. In this study, we used biochemical and genetic approaches to define the inhibitory features of the CHOP uORF and the biological consequences of loss of the CHOP uORF on CHOP expression during stress. We discovered that specific sequences within the CHOP uORF serve to stall elongating ribosomes and prevent ribosome reinitiation at the downstream CHOP coding sequence. As a consequence, deletion of the CHOP uORF substantially increases the levels and modifies the pattern of induction of CHOP expression in the ISR. Enhanced CHOP expression leads to increased expression of key CHOP target genes, culminating in increased cell death in response to stress.
Keywords: CHOP; DDIT3; GADD153; endoplasmic reticulum stress (ER stress); eukaryotic initiation factor 2 (eIF2); stress response; translation control; translation initiation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
An RNA stem-loop functions in conjunction with an upstream open reading frame to direct preferential translation in the integrated stress response.J Biol Chem. 2023 Feb;299(2):102864. doi: 10.1016/j.jbc.2022.102864. Epub 2022 Dec 31. J Biol Chem. 2023. PMID: 36596357 Free PMC article.
-
Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation.J Biol Chem. 2011 Apr 1;286(13):10939-49. doi: 10.1074/jbc.M110.216093. Epub 2011 Feb 1. J Biol Chem. 2011. PMID: 21285359 Free PMC article.
-
Ribosome Reinitiation Directs Gene-specific Translation and Regulates the Integrated Stress Response.J Biol Chem. 2015 Nov 20;290(47):28257-28271. doi: 10.1074/jbc.M115.693184. Epub 2015 Oct 7. J Biol Chem. 2015. PMID: 26446796 Free PMC article.
-
Upstream Open Reading Frames Differentially Regulate Gene-specific Translation in the Integrated Stress Response.J Biol Chem. 2016 Aug 12;291(33):16927-35. doi: 10.1074/jbc.R116.733899. Epub 2016 Jun 29. J Biol Chem. 2016. PMID: 27358398 Free PMC article. Review.
-
Surviving and Adapting to Stress: Translational Control and the Integrated Stress Response.Antioxid Redox Signal. 2023 Aug;39(4-6):351-373. doi: 10.1089/ars.2022.0123. Epub 2023 May 9. Antioxid Redox Signal. 2023. PMID: 36943285 Free PMC article. Review.
Cited by
-
Repeated Exposure to D-Amphetamine Decreases Global Protein Synthesis and Regulates the Translation of a Subset of mRNAs in the Striatum.Front Mol Neurosci. 2017 Jan 10;9:165. doi: 10.3389/fnmol.2016.00165. eCollection 2016. Front Mol Neurosci. 2017. PMID: 28119566 Free PMC article.
-
Identification of Arabidopsis thaliana upstream open reading frames encoding peptide sequences that cause ribosomal arrest.Nucleic Acids Res. 2017 Sep 6;45(15):8844-8858. doi: 10.1093/nar/gkx528. Nucleic Acids Res. 2017. PMID: 28637336 Free PMC article.
-
Deep transcriptome annotation enables the discovery and functional characterization of cryptic small proteins.Elife. 2017 Oct 30;6:e27860. doi: 10.7554/eLife.27860. Elife. 2017. PMID: 29083303 Free PMC article.
-
Incomplete Penetrance and Variable Expressivity: From Clinical Studies to Population Cohorts.Front Genet. 2022 Jul 25;13:920390. doi: 10.3389/fgene.2022.920390. eCollection 2022. Front Genet. 2022. PMID: 35983412 Free PMC article. Review.
-
An RNA stem-loop functions in conjunction with an upstream open reading frame to direct preferential translation in the integrated stress response.J Biol Chem. 2023 Feb;299(2):102864. doi: 10.1016/j.jbc.2022.102864. Epub 2022 Dec 31. J Biol Chem. 2023. PMID: 36596357 Free PMC article.
References
-
- Hinnebusch A. G. (2014) The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 83, 779–812 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

