Post-endocytotic Deubiquitination and Degradation of the Metabotropic γ-Aminobutyric Acid Receptor by the Ubiquitin-specific Protease 14
- PMID: 26817839
- PMCID: PMC4807296
- DOI: 10.1074/jbc.M115.686907
Post-endocytotic Deubiquitination and Degradation of the Metabotropic γ-Aminobutyric Acid Receptor by the Ubiquitin-specific Protease 14
Abstract
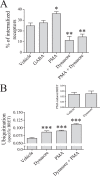
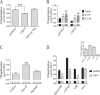
Mechanisms controlling the metabotropic γ-aminobutyric acid receptor (GABAB) cell surface stability are still poorly understood. In contrast with many other G protein-coupled receptors (GPCR), it is not subject to agonist-promoted internalization, but is constitutively internalized and rapidly down-regulated. In search of novel interacting proteins regulating receptor fate, we report that the ubiquitin-specific protease 14 (USP14) interacts with the GABAB(1b)subunit's second intracellular loop. Probing the receptor for ubiquitination using bioluminescence resonance energy transfer (BRET), we detected a constitutive and phorbol 12-myristate 13-acetate (PMA)-induced ubiquitination of the receptor at the cell surface. PMA also increased internalization and accelerated receptor degradation. Overexpression of USP14 decreased ubiquitination while treatment with a small molecule inhibitor of the deubiquitinase (IU1) increased receptor ubiquitination. Treatment with the internalization inhibitor Dynasore blunted both USP14 and IU1 effects on the receptor ubiquitination state, suggesting a post-endocytic site of action. Overexpression of USP14 also led to an accelerated degradation of GABABin a catalytically independent fashion. We thus propose a model whereby cell surface ubiquitination precedes endocytosis, after which USP14 acts as an ubiquitin-binding protein that targets the ubiquitinated receptor to lysosomal degradation and promotes its deubiquitination.
Keywords: G protein-coupled receptor (GPCR); GABA receptor; bioluminescence resonance energy transfer (BRET); biosensor; deubiquitylation (deubiquitination); protein degradation; receptor endocytosis; ubiquitylation (ubiquitination).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







Similar articles
-
Heterologous regulation of CXCR4 lysosomal trafficking.J Biol Chem. 2019 May 17;294(20):8023-8036. doi: 10.1074/jbc.RA118.005991. Epub 2019 Apr 1. J Biol Chem. 2019. PMID: 30936203 Free PMC article.
-
Lys-63-linked Ubiquitination of γ-Aminobutyric Acid (GABA), Type B1, at Multiple Sites by the E3 Ligase Mind Bomb-2 Targets GABAB Receptors to Lysosomal Degradation.J Biol Chem. 2016 Oct 7;291(41):21682-21693. doi: 10.1074/jbc.M116.750968. Epub 2016 Aug 29. J Biol Chem. 2016. PMID: 27573246 Free PMC article.
-
The deubiquitinating enzyme Usp14 allosterically inhibits multiple proteasomal activities and ubiquitin-independent proteolysis.J Biol Chem. 2017 Jun 9;292(23):9830-9839. doi: 10.1074/jbc.M116.763128. Epub 2017 Apr 17. J Biol Chem. 2017. PMID: 28416611 Free PMC article.
-
Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination.Cell Biochem Biophys. 2011 Jun;60(1-2):39-46. doi: 10.1007/s12013-011-9181-9. Cell Biochem Biophys. 2011. PMID: 21448666 Review.
-
Emerging role of ubiquitin-specific protease 14 in oncogenesis and development of tumor: Therapeutic implication.Life Sci. 2019 Dec 15;239:116875. doi: 10.1016/j.lfs.2019.116875. Epub 2019 Oct 30. Life Sci. 2019. PMID: 31676235 Review.
Cited by
-
Diversity of structure and function of GABAB receptors: a complexity of GABAB-mediated signaling.Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(10):390-411. doi: 10.2183/pjab.94.026. Proc Jpn Acad Ser B Phys Biol Sci. 2018. PMID: 30541966 Free PMC article. Review.
-
Regulation of G Protein-Coupled Receptors by Ubiquitination.Int J Mol Sci. 2017 Apr 27;18(5):923. doi: 10.3390/ijms18050923. Int J Mol Sci. 2017. PMID: 28448471 Free PMC article. Review.
-
Post-Translational Modifications of G Protein-Coupled Receptors Control Cellular Signaling Dynamics in Space and Time.Pharmacol Rev. 2021 Jan;73(1):120-151. doi: 10.1124/pharmrev.120.000082. Pharmacol Rev. 2021. PMID: 33268549 Free PMC article. Review.
-
Development of a Novel SNAP-Epitope Tag/Near-Infrared Imaging Assay to Quantify G Protein-Coupled Receptor Degradation in Human Cells.SLAS Discov. 2021 Apr;26(4):570-578. doi: 10.1177/2472555220979793. Epub 2021 Jan 5. SLAS Discov. 2021. PMID: 33402011 Free PMC article.
-
Agonist-activated glucagon receptors are deubiquitinated at early endosomes by two distinct deubiquitinases to facilitate Rab4a-dependent recycling.J Biol Chem. 2020 Dec 4;295(49):16630-16642. doi: 10.1074/jbc.RA120.014532. Epub 2020 Sep 23. J Biol Chem. 2020. PMID: 32967969 Free PMC article.
References
-
- Luttrell L. M., and Lefkowitz R. J. (2002) The role of {beta}-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 115, 455–465 - PubMed
-
- White J. H., Wise a, Main M. J., Green A., Fraser N. J., Disney G. H., Barnes A. A., Emson P., Foord S. M., and Marshall F. H. (1998) Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396, 679–682 - PubMed
-
- Kanaide M., and Uezono Y. (2007) Desensitization of GABAB receptor signaling by formation of protein complexes of GABAB2 subunit with GRK4 or GRK5. J. Cell Physiol. 210, 237–245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

