Post-endocytotic Deubiquitination and Degradation of the Metabotropic γ-Aminobutyric Acid Receptor by the Ubiquitin-specific Protease 14
- PMID: 26817839
- PMCID: PMC4807296
- DOI: 10.1074/jbc.M115.686907
Post-endocytotic Deubiquitination and Degradation of the Metabotropic γ-Aminobutyric Acid Receptor by the Ubiquitin-specific Protease 14
Abstract
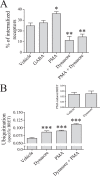
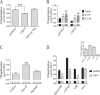
Mechanisms controlling the metabotropic γ-aminobutyric acid receptor (GABAB) cell surface stability are still poorly understood. In contrast with many other G protein-coupled receptors (GPCR), it is not subject to agonist-promoted internalization, but is constitutively internalized and rapidly down-regulated. In search of novel interacting proteins regulating receptor fate, we report that the ubiquitin-specific protease 14 (USP14) interacts with the GABAB(1b)subunit's second intracellular loop. Probing the receptor for ubiquitination using bioluminescence resonance energy transfer (BRET), we detected a constitutive and phorbol 12-myristate 13-acetate (PMA)-induced ubiquitination of the receptor at the cell surface. PMA also increased internalization and accelerated receptor degradation. Overexpression of USP14 decreased ubiquitination while treatment with a small molecule inhibitor of the deubiquitinase (IU1) increased receptor ubiquitination. Treatment with the internalization inhibitor Dynasore blunted both USP14 and IU1 effects on the receptor ubiquitination state, suggesting a post-endocytic site of action. Overexpression of USP14 also led to an accelerated degradation of GABABin a catalytically independent fashion. We thus propose a model whereby cell surface ubiquitination precedes endocytosis, after which USP14 acts as an ubiquitin-binding protein that targets the ubiquitinated receptor to lysosomal degradation and promotes its deubiquitination.
Keywords: G protein-coupled receptor (GPCR); GABA receptor; bioluminescence resonance energy transfer (BRET); biosensor; deubiquitylation (deubiquitination); protein degradation; receptor endocytosis; ubiquitylation (ubiquitination).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Luttrell L. M., and Lefkowitz R. J. (2002) The role of {beta}-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 115, 455–465 - PubMed
-
- White J. H., Wise a, Main M. J., Green A., Fraser N. J., Disney G. H., Barnes A. A., Emson P., Foord S. M., and Marshall F. H. (1998) Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396, 679–682 - PubMed
-
- Kanaide M., and Uezono Y. (2007) Desensitization of GABAB receptor signaling by formation of protein complexes of GABAB2 subunit with GRK4 or GRK5. J. Cell Physiol. 210, 237–245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

