Structural Basis for the Inhibition of Voltage-gated Sodium Channels by Conotoxin μO§-GVIIJ
- PMID: 26817840
- PMCID: PMC4807300
- DOI: 10.1074/jbc.M115.697672
Structural Basis for the Inhibition of Voltage-gated Sodium Channels by Conotoxin μO§-GVIIJ
Abstract
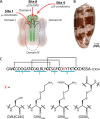
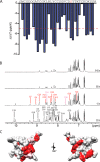
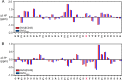
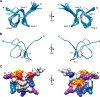
Cone snail toxins are well known blockers of voltage-gated sodium channels, a property that is of broad interest in biology and therapeutically in treating neuropathic pain and neurological disorders. Although most conotoxin channel blockers function by direct binding to a channel and disrupting its normal ion movement, conotoxin μO§-GVIIJ channel blocking is unique, using both favorable binding interactions with the channel and a direct tether via an intermolecular disulfide bond. Disulfide exchange is possible because conotoxin μO§-GVIIJ contains anS-cysteinylated Cys-24 residue that is capable of exchanging with a free cysteine thiol on the channel surface. Here, we present the solution structure of an analog of μO§-GVIIJ (GVIIJ[C24S]) and the results of structure-activity studies with synthetic μO§-GVIIJ variants. GVIIJ[C24S] adopts an inhibitor cystine knot structure, with two antiparallel β-strands stabilized by three disulfide bridges. The loop region linking the β-strands (loop 4) presents residue 24 in a configuration where it could bind to the proposed free cysteine of the channel (Cys-910, rat NaV1.2 numbering; at site 8). The structure-activity study shows that three residues (Lys-12, Arg-14, and Tyr-16) located in loop 2 and spatially close to residue 24 were also important for functional activity. We propose that the interaction of μO§-GVIIJ with the channel depends on not only disulfide tethering via Cys-24 to a free cysteine at site 8 on the channel but also the participation of key residues of μO§-GVIIJ on a distinct surface of the peptide.
Keywords: conotoxin; cysteine; disulfide; high performance liquid chromatography (HPLC); nuclear magnetic resonance (NMR); peptide chemical synthesis; sodium channel; structure-activity relationship studies; two-electrode voltage clamp electrophysiology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Probing the Redox States of Sodium Channel Cysteines at the Binding Site of μO§-Conotoxin GVIIJ.Biochemistry. 2015 Jun 30;54(25):3911-20. doi: 10.1021/acs.biochem.5b00390. Epub 2015 Jun 18. Biochemistry. 2015. PMID: 26039939
-
A disulfide tether stabilizes the block of sodium channels by the conotoxin μO§-GVIIJ.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2758-63. doi: 10.1073/pnas.1324189111. Epub 2014 Feb 4. Proc Natl Acad Sci U S A. 2014. PMID: 24497506 Free PMC article.
-
Α- and β-subunit composition of voltage-gated sodium channels investigated with μ-conotoxins and the recently discovered μO§-conotoxin GVIIJ.J Neurophysiol. 2015 Apr 1;113(7):2289-301. doi: 10.1152/jn.01004.2014. Epub 2015 Jan 28. J Neurophysiol. 2015. PMID: 25632083 Free PMC article.
-
Conotoxin modulation of voltage-gated sodium channels.Int J Biochem Cell Biol. 2008;40(11):2363-8. doi: 10.1016/j.biocel.2007.08.017. Epub 2007 Sep 14. Int J Biochem Cell Biol. 2008. PMID: 17951097 Review.
-
Structure and function of μ-conotoxins, peptide-based sodium channel blockers with analgesic activity.Future Med Chem. 2014 Oct;6(15):1677-98. doi: 10.4155/fmc.14.107. Future Med Chem. 2014. PMID: 25406007 Free PMC article. Review.
Cited by
-
Conotoxins Targeting Voltage-Gated Sodium Ion Channels.Pharmacol Rev. 2024 Aug 15;76(5):828-845. doi: 10.1124/pharmrev.123.000923. Pharmacol Rev. 2024. PMID: 38914468 Free PMC article. Review.
-
µ-Conotoxins Modulating Sodium Currents in Pain Perception and Transmission: A Therapeutic Potential.Mar Drugs. 2017 Sep 22;15(10):295. doi: 10.3390/md15100295. Mar Drugs. 2017. PMID: 28937587 Free PMC article. Review.
-
A Transcriptomic Survey of Ion Channel-Based Conotoxins in the Chinese Tubular Cone Snail (Conus betulinus).Mar Drugs. 2017 Jul 18;15(7):228. doi: 10.3390/md15070228. Mar Drugs. 2017. PMID: 28718820 Free PMC article.
References
-
- Leipold E., DeBie H., Zorn S., Borges A., Olivera B. M., Terlau H., and Heinemann S. H. (2007) μO-conotoxins inhibit NaV channels by interfering with their voltage sensors in domain-2. Channels 1, 253–262 - PubMed
-
- Gajewiak J., Azam L., Imperial J., Walewska A., Green B. R., Bandyopadhyay P. K., Raghuraman S., Ueberheide B., Bern M., Zhou H. M., Minassian N. A., Hagan R. H., Flinspach M., Liu Y., Bulaj G., Wickenden A. D., Olivera B. M., Yoshikami D., and Zhang M. M. (2014) A disulfide tether stabilizes the block of sodium channels by the conotoxin μO§-GVIIJ. Proc. Natl. Acad. Sci. U.S.A. 111, 2758–2763 - PMC - PubMed
-
- Zhang M. M., Gajewiak J., Azam L., Bulaj G., Olivera B. M., and Yoshikami D. (2015) Probing the redox states of sodium channel cysteines at the binding site of μO§-conotoxin GVIIJ. Biochemistry 54, 3911–3920 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

