A Phase II Study of Sequential Capecitabine Plus Oxaliplatin Followed by Docetaxel Plus Capecitabine in Patients With Unresectable Gastric Adenocarcinoma: The TCOG 3211 Clinical Trial
- PMID: 26817912
- PMCID: PMC4998286
- DOI: 10.1097/MD.0000000000002565
A Phase II Study of Sequential Capecitabine Plus Oxaliplatin Followed by Docetaxel Plus Capecitabine in Patients With Unresectable Gastric Adenocarcinoma: The TCOG 3211 Clinical Trial
Abstract
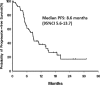
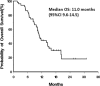
Fluorouracil and platinum are considered the standard treatment options for advanced gastric cancer. Docetaxel is also an effective agent and it shows no cross-resistance with fluorouracil and platinum. The combination treatment of docetaxel with fluorouracil and platinum has been explored, but it demonstrated intolerable toxicities. An alternative approach in the first-line treatment of gastric adenocarcinoma may be to use these agents sequentially. We aimed to evaluate the activity and safety profile of sequential chemotherapy with capecitabine plus oxaliplatin, followed by docetaxel plus capecitabine in the first-line treatment of unresectable gastric cancer.We conducted a phase II study of sequential first-line chemotherapy in advanced gastric cancer. Treatment consisted of 6 cycles of capecitabine plus oxaliplatin (capecitabine 1000 mg/m bid on days 1-10 and oxaliplatin 85 mg/m on day 1, every 2 weeks), followed by 4 cycles of docetaxel plus capecitabine (docetaxel 30 mg/m on days 1 and 8, capecitabine 825 mg/m bid on days 1-14, every 3 weeks). The primary end-point was the objective response rate.Fifty-one patients were enrolled: median age, 63 years; male/female: 37/14. The main grade 3 to 4 toxicities were a decreased absolute neutrophil count (25.4%), diarrhea (9.8%), and hand-foot syndrome (15.7%). The objective response rate was 61.7%. The median progression-free survival and overall survival were 8.6 and 11.0 months, respectively. Six patients (11.8%) received surgery after chemotherapy and 5 are still disease-free.This sequential treatment demonstrated feasibility with a favorable safety profile and produced encouraging results in terms of activity and efficacy.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 2013; 14:e535–e547. - PubMed
-
- Kuo CY, Chao Y, Li CP. Update on treatment of gastric cancer. J Chin Med Assoc 2014; 77:345–353. - PubMed
-
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010; 3:CD004064. - PubMed
-
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24:2903–2909. - PubMed
-
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24:4991–4997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

