Near real-time vaccine safety surveillance using electronic health records-a systematic review of the application of statistical methods
- PMID: 26817940
- PMCID: PMC5021108
- DOI: 10.1002/pds.3966
Near real-time vaccine safety surveillance using electronic health records-a systematic review of the application of statistical methods
Abstract
Purpose: Pre-licensure studies have limited ability to detect rare adverse events (AEs) to vaccines, requiring timely post-licensure studies. With the increasing availability of electronic health records (EHR) near real-time vaccine safety surveillance using these data has emerged as an option. We reviewed methods currently used to inform development of similar systems for countries considering their introduction.
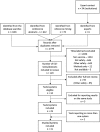
Methods: Medline, EMBASE and Web of Science were searched, with additional searches of conference abstract books. Questionnaires were sent to organizations worldwide to ascertain unpublished studies. Eligible studies used EHR and regularly assessed pre-specified AE to vaccine(s). Key features of studies were compared descriptively.
Results: From 2779 studies, 31 were included from the USA (23), UK (6), and Taiwan and New Zealand (1 each). These were published/conducted between May 2005 and April 2015. Thirty-eight different vaccines were studied, focusing mainly on influenza (47.4%), especially 2009 H1N1 vaccines. Forty-six analytic approaches were used, reflecting frequency of EHR updates and the AE studied. Poisson-based maximized sequential probability ratio test was the most common (43.5%), followed by its binomial (23.9%) and conditional versions (10.9%). Thirty-seven of 49 analyses (75.5%) mentioned control for confounding, using an adjusted expected rate (51.4% of those adjusting), stratification (16.2%) or a combination of a self-controlled design and stratification (13.5%). Guillain-Barré syndrome (11.9%), meningitis/encephalitis/myelitis (11.9%) and seizures (10.8%) were studied most often.
Conclusions: Near real-time vaccine safety surveillance using EHR has developed over the past decade but is not yet widely used. As more countries have access to EHR, it will be important that appropriate methods are selected, considering the data available and AE of interest.
Keywords: electronic health records; pharmacoepidemiology; safety; sequential tests; statistical process control; vaccines.
© 2016 The Authors. Pharmacoepidemiology and Drug Safety Published by John Wiley & Sons Ltd.
Figures

References
-
- Bonhoeffer J, Black S, Izurieta H, Zuber P, Sturkenboom M. Current status and future directions of post‐marketing vaccine safety monitoring with focus on USA and Europe. Biologicals 2012 Sep; 40: 393–397. - PubMed
-
- Chen RT, Glanz JM, Vellozzi C. Pharmacoepidemiologic studies of vaccine safety In Pharmacoepidemiology. Strom BL, Kimmel SE. and Hennessy S. (eds). Wiley‐Backwell: Chicester, 2011; 423–468.
-
- McPhillips H, Marcuse EK. Vaccine safety. Curr Probl Pediatr 2001; 31: 95–121. - PubMed
-
- Lopalco PL, Johansen K, Ciancio B, Gomes HC, Kramarz P, Giesecke J. Monitoring and assessing vaccine safety: a European perspective. Expert Rev Vaccines 2010; 9: 371–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

