Mechanisms of sex determination and transmission ratio distortion in Aedes aegypti
- PMID: 26818000
- PMCID: PMC4730765
- DOI: 10.1186/s13071-016-1331-x
Mechanisms of sex determination and transmission ratio distortion in Aedes aegypti
Abstract
Background: More effective mosquito control strategies are urgently required due to the increasing prevalence of insecticide resistance. The sterile insect technique (SIT) and the release of insects carrying a dominant lethal allele (RIDL) are two proposed methods for environmentally-friendly, species-targeted population control. These methods may be more suitable for developing countries if producers reduce the cost of rearing insects. The cost of control programs could be reduced by producing all-male mosquito populations to circumvent the isolation of females before release without reducing male mating competitiveness caused by transgenes.
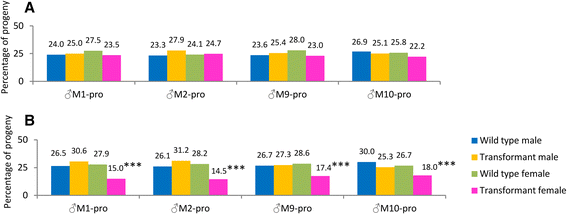
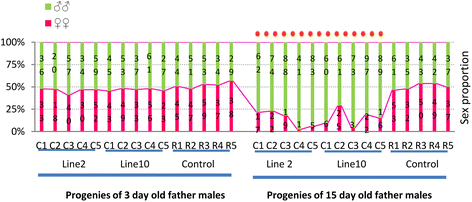
Results: An RNAi construct targeting the RNA recognition motif of the Aedes aegypti transformer-2 (tra-2) gene does not trigger female-to-male sex conversion as commonly observed among dipterous insects. Instead, homozygous insects show greater mortality among m-chromosome-bearing sperm and mm zygotes, yielding up to 100% males in the subsequent generations. The performance of transgenic males was not significantly different to wild-type males in narrow-cage competitive mating experiments.
Conclusion: Our data provide preliminary evidence that the knockdown of Ae. aegypti tra-2 gene expression causes segregation distortion acting at the level of gametic function, which is reinforced by sex-specific zygotic lethality. This finding could promote the development of new synthetic sex distorter systems for the production of genetic sexing mosquito strains.
Figures




References
-
- Knipling E. Possibilities of insect control or eradication through use of sexually sterile males. J Econ Entomol. 1955;48:459–62. doi: 10.1093/jee/48.4.459. - DOI
-
- Winskill P, Harris AF, Morgan SA, Stevenson J, Raduan N, Alphey L, et al. Genetic control of Aedes aegypti: data-driven modelling to assess the effect of releasing different life stages and the potential for long-term suppression. Parasites Vectors. 2014;7:68. doi: 10.1186/1756-3305-7-68. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

