Studies on human eRF3-PABP interaction reveal the influence of eRF3a N-terminal glycin repeat on eRF3-PABP binding affinity and the lower affinity of eRF3a 12-GGC allele involved in cancer susceptibility
- PMID: 26818177
- PMCID: PMC4829321
- DOI: 10.1080/15476286.2015.1137421
Studies on human eRF3-PABP interaction reveal the influence of eRF3a N-terminal glycin repeat on eRF3-PABP binding affinity and the lower affinity of eRF3a 12-GGC allele involved in cancer susceptibility
Abstract
The eukaryotic release factor 3 (eRF3) has been involved in the control of mRNA degradation through its association with the cytoplasmic Poly(A) Binding Protein, PABP. In mammals, eRF3 N-terminal domain contains two overlapping PAM2 motifs which specifically recognize the MLLE domain of PABP. In humans, eRF3a/GSPT1 gene contains a stable GGC repeat encoding a repeat of glycine residues in eRF3a N-terminus. There are five known eRF3a/GSPT1 alleles in the human population, encoding 7, 9, 10, 11 and 12 glycines. Several studies have reported that the presence of eRF3a 12-GGC allele is correlated with an increased risk of cancer development. Using surface plasmon resonance, we have studied the interaction of the various allelic forms of eRF3a with PABP alone or poly(A)-bound PABP. We found that the N-terminal glycine repeat of eRF3a influences eRF3a-PABP interaction and that eRF3a 12-GGC allele has a decreased binding affinity for PABP. Our comparative analysis on eRF3a alleles suggests that the presence of eRF3a 12-GGC allele could modify the coupling between translation termination and mRNA deadenylation.
Keywords: Cancer; GSPT1; GSPT2; PABP; eRF3; mRNA degradation; mRNA poly(A) tail; surface plasmon resonance; translation termination.
Figures
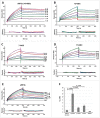
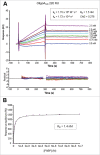
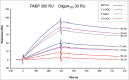

References
-
- Cosson B, Berkova N, Couturier A, Chabelskaya S, Philippe M, Zhouravleva G. Poly(A)-binding protein and eRF3 are associated in vivo in human and Xenopus cells. Biol Cell 2002; 94:205-16; PMID:12489690; http://dx.doi.org/10.1016/S0248-4900(02)01194-2 - DOI - PubMed
-
- Hoshino S, Imai M, Kobayashi T, Uchida N, Katada T. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-Poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J Biol Chem 1999; 274:16677-80; PMID:10358005; http://dx,doi.org/10.1074/jbc.274.24.16677 - PubMed
-
- Hoshino S. Mechanism of the initiation of mRNA decay: role of eRF3 family G proteins. Wiley Interdiscip Rev RNA 2012; 3:743-57; PMID:22965901; http://dx.doi.org/10.1002/wrna.1133 - DOI - PubMed
-
- Burgess HM, Gray NK. mRNA-specific regulation of translation by poly(A)-binding proteins. Biochem Soc Trans 2010; 38:1517-22; PMID:21118118; http://dx.doi.org/10.1042/BST0381517 - DOI - PubMed
-
- Bernstein P, Peltz SW, Ross J. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol 1989; 9:659-70; PMID:2565532; http://dx.doi.org/10.1128/MCB.9.2.659 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
