Wnt11 plays an important role in the osteogenesis of human mesenchymal stem cells in a PHA/FN/ALG composite scaffold: possible treatment for infected bone defect
- PMID: 26818191
- PMCID: PMC4729148
- DOI: 10.1186/s13287-016-0277-4
Wnt11 plays an important role in the osteogenesis of human mesenchymal stem cells in a PHA/FN/ALG composite scaffold: possible treatment for infected bone defect
Abstract
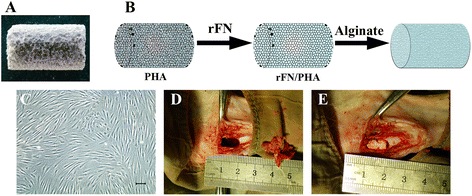
Background: Infected bone defect poses a great challenge for orthopedists because it is difficult to cure. Tissue-engineered bone based on the human mesenchymal stem cells (hMSCs), has currently taken a promising treatment protocol in clinical practice. In a previous study, a porous hydroxyapatite/fibronectin/alginate (PHA/FN/ALG) composite scaffold displayed favorable biological properties as a novel scaffold, which was considered better than single-material scaffolds. In addition, Wnt11 has been demonstrated to play an important role in the development of osteoblasts, but until recently, its role in the osteogenic differentiation of hMSCs in infectious environment remained unclear.
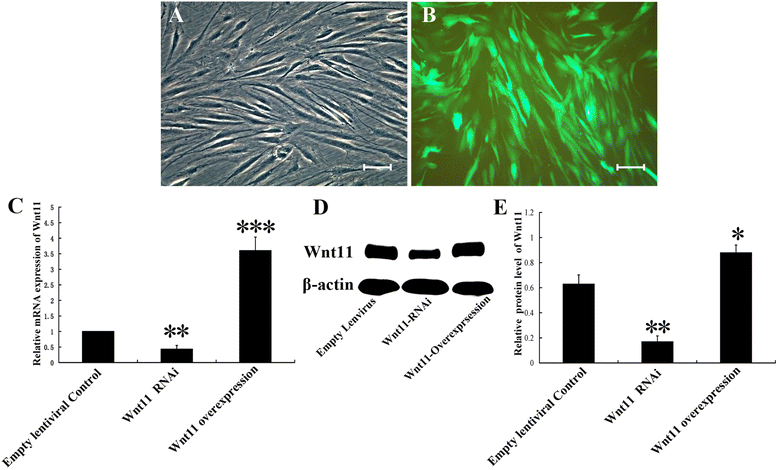
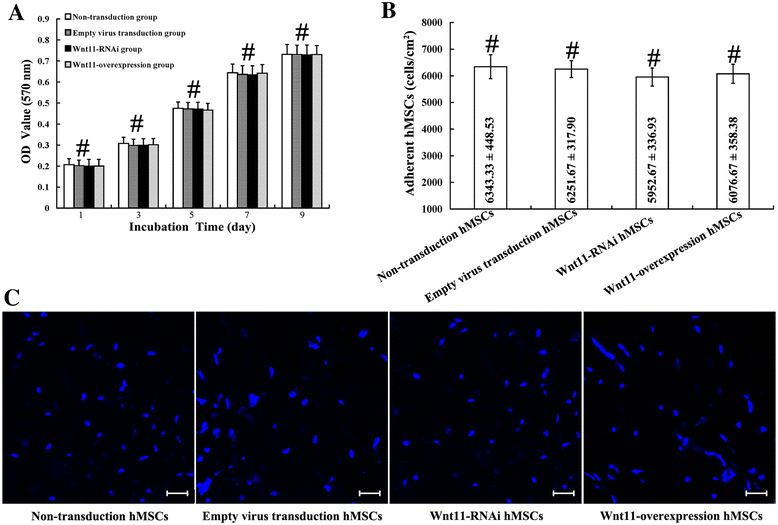
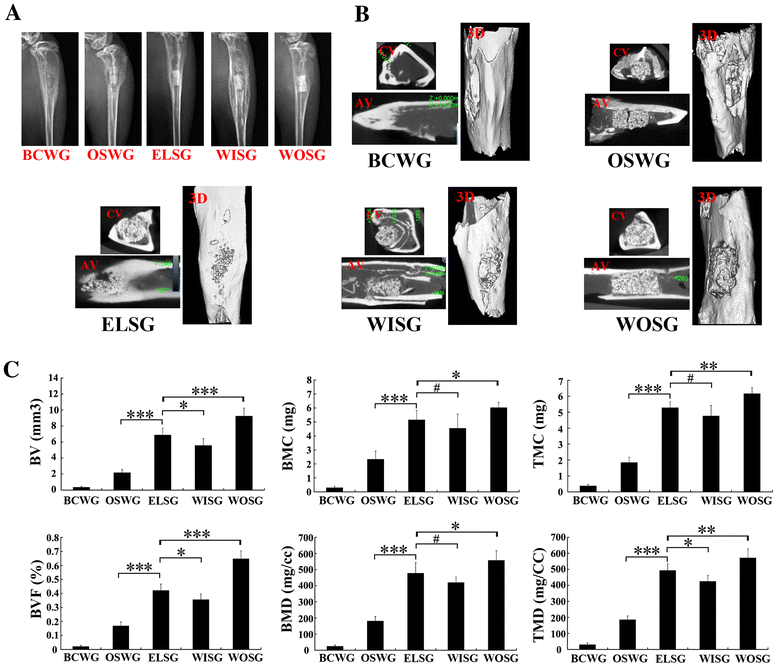
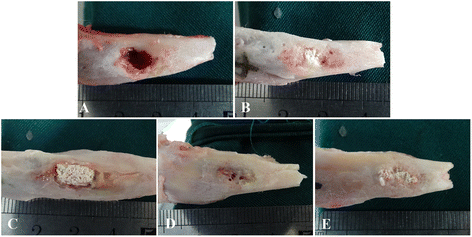
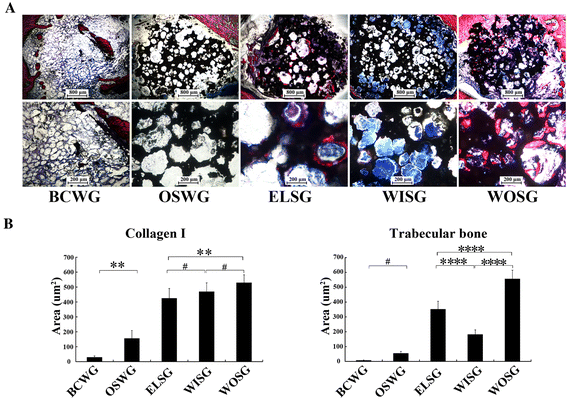
Methods: In this study, we constructed a PHA/FN/ALG composite scaffold with layer-by-layer technology. Furthermore, we also constructed Wnt11-silenced (RNAi) and -overexpressing hMSCs by lentiviral transduction. The gene transduction efficacy was confirmed by quantitative PCR assay and Western blot analysis. Tissue-engineered bone was constructed with hMSCs and PHA/FN/ALG composite scaffolds, and then was implanted into an infected bone defect model for evaluating the osteogenic capacity by quantitative PCR, gross observation, micro-CT and histology analysis.
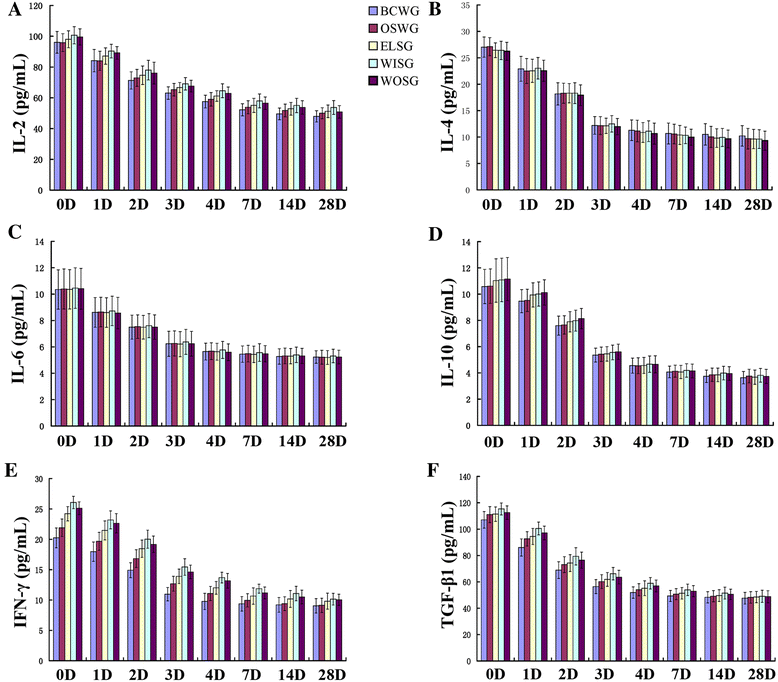
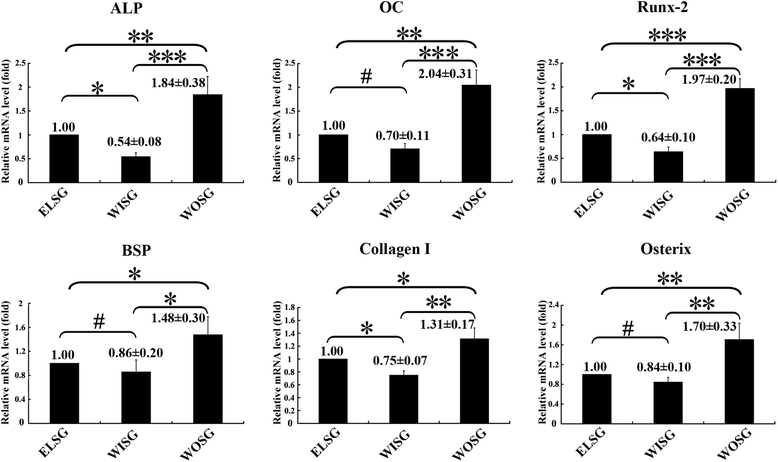
Results: All those cells showed similar adhesion abilities and proliferation capacities in scaffolds. After tissue-engineered bone implantation, there were high levels of systemic inflammatory factors in vivo, which significantly declined three days after antibiotic therapy. One or two months after implantation, the results of osteogenic-related gene analyses, gross observation, micro-CT and histology consistently showed that the Wnt11 over-expression hMSC group displayed the strongest osteogenesis capacity, whereas the Wnt11-RNAi hMSC group displayed inferior osteogenesis capacity, when compared with the other cell-containing groups. However, the blank control group and the only composite scaffold without cell implantation group both showed extremely weak osteogenesis capacity.
Conclusion: Our results revealed that the Wnt11 gene plays an important role in hMSCs for enhancing the osteogenesis in an infectious environment.
Figures
Similar articles
-
Octacalcium phosphate-precipitated alginate scaffold for bone regeneration.Tissue Eng Part A. 2009 Nov;15(11):3525-35. doi: 10.1089/ten.TEA.2009.0048. Tissue Eng Part A. 2009. PMID: 19456237
-
3D-Printed Atsttrin-Incorporated Alginate/Hydroxyapatite Scaffold Promotes Bone Defect Regeneration with TNF/TNFR Signaling Involvement.Adv Healthc Mater. 2015 Aug 5;4(11):1701-8. doi: 10.1002/adhm.201500211. Epub 2015 Jun 17. Adv Healthc Mater. 2015. PMID: 26085382
-
Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering.Acta Biomater. 2011 Aug;7(8):3178-86. doi: 10.1016/j.actbio.2011.04.008. Epub 2011 Apr 27. Acta Biomater. 2011. PMID: 21539944
-
The response of human mesenchymal stem cells to osteogenic signals and its impact on bone tissue engineering.Curr Stem Cell Res Ther. 2007 Sep;2(3):209-20. doi: 10.2174/157488807781696267. Curr Stem Cell Res Ther. 2007. PMID: 18220904 Review.
-
Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance.Curr Stem Cell Res Ther. 2008 Dec;3(4):254-64. doi: 10.2174/157488808786733962. Curr Stem Cell Res Ther. 2008. PMID: 19075755 Free PMC article. Review.
Cited by
-
Osteoblast-oriented differentiation of BMSCs by co-culturing with composite scaffolds constructed using silicon-substituted calcium phosphate, autogenous fine particulate bone powder and alginate in vitro.Oncotarget. 2017 Jul 5;8(51):88308-88319. doi: 10.18632/oncotarget.19015. eCollection 2017 Oct 24. Oncotarget. 2017. PMID: 29179436 Free PMC article.
-
Reconstructive Science in Orthopedic Oncology.Tech Orthop. 2018 Sep;33(3):175-182. doi: 10.1097/BTO.0000000000000282. Tech Orthop. 2018. PMID: 30636842 Free PMC article.
-
Macrophage inhibits the osteogenesis of fibroblasts in ultrahigh molecular weight polyethylene (UHMWPE) wear particle-induced osteolysis.J Orthop Surg Res. 2019 Mar 18;14(1):80. doi: 10.1186/s13018-019-1119-8. J Orthop Surg Res. 2019. PMID: 30885228 Free PMC article.
-
Decoding the transcriptome of calcified atherosclerotic plaque at single-cell resolution.Commun Biol. 2022 Oct 12;5(1):1084. doi: 10.1038/s42003-022-04056-7. Commun Biol. 2022. PMID: 36224302 Free PMC article.
-
Micro-CT - a digital 3D microstructural voyage into scaffolds: a systematic review of the reported methods and results.Biomater Res. 2018 Sep 26;22:26. doi: 10.1186/s40824-018-0136-8. eCollection 2018. Biomater Res. 2018. PMID: 30275969 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

