Enhanced performance of macrophage-encapsulated nanoparticle albumin-bound-paclitaxel in hypo-perfused cancer lesions
- PMID: 26818212
- PMCID: PMC4919151
- DOI: 10.1039/c5nr07796f
Enhanced performance of macrophage-encapsulated nanoparticle albumin-bound-paclitaxel in hypo-perfused cancer lesions
Abstract
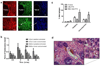
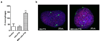
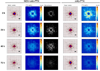
Hypovascularization in tumors such as liver metastases originating from breast and other organs correlates with poor chemotherapeutic response and higher mortality. Poor prognosis is linked to impaired transport of both low- and high-molecular weight drugs into the lesions and to high washout rate. Nanoparticle albumin-bound-paclitaxel (nAb-PTX) has demonstrated benefits in clinical trials when compared to paclitaxel and docetaxel. However, its therapeutic efficacy for breast cancer liver metastasis is disappointing. As macrophages are the most abundant cells in the liver tumor microenvironment, we design a multistage system employing macrophages to deliver drugs into hypovascularized metastatic lesions, and perform in vitro, in vivo, and in silico evaluation. The system encapsulates nAb-PTX into nanoporous biocompatible and biodegradable multistage vectors (MSV), thus promoting nAb-PTX retention in macrophages. We develop a 3D in vitro model to simulate clinically observed hypo-perfused tumor lesions surrounded by macrophages. This model enables evaluation of nAb-PTX and MSV-nab PTX efficacy as a function of transport barriers. Addition of macrophages to this system significantly increases MSV-nAb-PTX efficacy, revealing the role of macrophages in drug transport. In the in vivo model, a significant increase in macrophage number, as compared to unaffected liver, is observed in mice, confirming the in vitro findings. Further, a mathematical model linking drug release and retention from macrophages is implemented to project MSV-nAb-PTX efficacy in a clinical setting. Based on macrophage presence detected via liver tumor imaging and biopsy, the proposed experimental/computational approach could enable prediction of MSV-nab PTX performance to treat metastatic cancer in the liver.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

