Why Don't We Move Slower? The Value of Time in the Neural Control of Action
- PMID: 26818497
- PMCID: PMC6604817
- DOI: 10.1523/JNEUROSCI.1921-15.2016
Why Don't We Move Slower? The Value of Time in the Neural Control of Action
Abstract
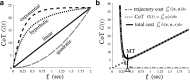
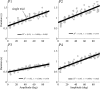
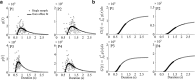
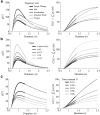
To want something now rather than later is a common attitude that reflects the brain's tendency to value the passage of time. Because the time taken to accomplish an action inevitably delays task achievement and reward acquisition, this idea was ported to neural movement control within the "cost of time" theory. This theory provides a normative framework to account for the underpinnings of movement time formation within the brain and the origin of a self-selected pace in human and animal motion. Then, how does the brain exactly value time in the control of action? To tackle this issue, we used an inverse optimal control approach and developed a general methodology allowing to squarely sample infinitesimal values of the time cost from experimental motion data. The cost of time underlying saccades was found to have a concave growth, thereby confirming previous results on hyperbolic reward discounting, yet without making any prior assumption about this hypothetical nature. For self-paced reaching, however, movement time was primarily valued according to a striking sigmoidal shape; its rate of change consistently presented a steep rise before a maximum was reached and a slower decay was observed. Theoretical properties of uniqueness and robustness of the inferred time cost were established for the class of problems under investigation, thus reinforcing the significance of the present findings. These results may offer a unique opportunity to uncover how the brain values the passage of time in healthy and pathological motor control and shed new light on the processes underlying action invigoration.
Significance statement: Movement time is a fundamental characteristic of neural motor control, but the principles underlying its formation remain little known. This work addresses that question within the inverse optimal control framework where the challenge is to uncover what optimality criterion underlies a system's behavior. Here we rely on the "cost of time" theory that finds its roots into the brain's tendency to discount the actual value of future reward. It asserts that the time elapsed until action completion entails a cost, thereby making slow moves nonoptimal. By means of a thorough theoretical analysis, the present article shows how to sample the infinitesimal values of the time cost without prior assumption about its hypothetical nature and emphasizes its sigmoidal shape for reaching.
Keywords: action vigor; cost of time; movement time; optimal control; reaching; saccade.
Copyright © 2016 the authors 0270-6474/16/361056-15$15.00/0.
Figures




Similar articles
-
Temporal discounting of reward and the cost of time in motor control.J Neurosci. 2010 Aug 4;30(31):10507-16. doi: 10.1523/JNEUROSCI.1343-10.2010. J Neurosci. 2010. PMID: 20685993 Free PMC article.
-
Evidence for a cost of time in the invigoration of isometric reaching movements.J Neurophysiol. 2022 Mar 1;127(3):689-701. doi: 10.1152/jn.00536.2021. Epub 2022 Feb 9. J Neurophysiol. 2022. PMID: 35138953
-
Vigor of movements and the cost of time in decision making.J Neurosci. 2014 Jan 22;34(4):1212-23. doi: 10.1523/JNEUROSCI.2798-13.2014. J Neurosci. 2014. PMID: 24453313 Free PMC article.
-
Control of movements and temporal discounting of reward.Curr Opin Neurobiol. 2010 Dec;20(6):726-30. doi: 10.1016/j.conb.2010.08.017. Curr Opin Neurobiol. 2010. PMID: 20833031 Review.
-
Movement Vigor as a Reflection of Subjective Economic Utility.Trends Neurosci. 2019 May;42(5):323-336. doi: 10.1016/j.tins.2019.02.003. Epub 2019 Mar 13. Trends Neurosci. 2019. PMID: 30878152 Free PMC article. Review.
Cited by
-
Understanding voluntary human movement variability through data-driven segmentation and clustering.Front Hum Neurosci. 2023 Nov 28;17:1278653. doi: 10.3389/fnhum.2023.1278653. eCollection 2023. Front Hum Neurosci. 2023. PMID: 38090552 Free PMC article.
-
Dissociating the Impact of Movement Time and Energy Costs on Decision-Making and Action Initiation in Humans.Front Hum Neurosci. 2021 Nov 1;15:715212. doi: 10.3389/fnhum.2021.715212. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34790104 Free PMC article.
-
Reinforcement learning control of a biomechanical model of the upper extremity.Sci Rep. 2021 Jul 14;11(1):14445. doi: 10.1038/s41598-021-93760-1. Sci Rep. 2021. PMID: 34262081 Free PMC article.
-
Time-order-errors and duration ranges in the Episodic Temporal Generalization task.Sci Rep. 2017 Jun 1;7(1):2643. doi: 10.1038/s41598-017-02386-9. Sci Rep. 2017. PMID: 28572663 Free PMC article.
-
Unifying Speed-Accuracy Trade-Off and Cost-Benefit Trade-Off in Human Reaching Movements.Front Hum Neurosci. 2017 Dec 19;11:615. doi: 10.3389/fnhum.2017.00615. eCollection 2017. Front Hum Neurosci. 2017. PMID: 29379424 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
