Investigating the Stability of Fine-Grain Digit Somatotopy in Individual Human Participants
- PMID: 26818501
- PMCID: PMC4728720
- DOI: 10.1523/JNEUROSCI.1742-15.2016
Investigating the Stability of Fine-Grain Digit Somatotopy in Individual Human Participants
Abstract
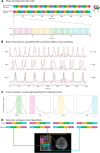
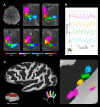
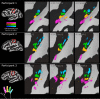
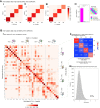
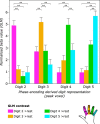
Studies of human primary somatosensory cortex (S1) have placed a strong emphasis on the cortical representation of the hand and the propensity for plasticity therein. Despite many reports of group differences and experience-dependent changes in cortical digit somatotopy, relatively little work has considered the variability of these maps across individuals and to what extent this detailed functional architecture is dynamic over time. With the advent of 7 T fMRI, it is increasingly feasible to map such detailed organization noninvasively in individual human participants. Here, we extend the ability of ultra-high-field imaging beyond a technological proof of principle to investigate the intersubject variability of digit somatotopy across participants and the stability of this organization across a range of intervals. Using a well validated phase-encoding paradigm and an active task, we demonstrate the presence of highly reproducible maps of individual digits in S1, sharply contrasted by a striking degree of intersubject variability in the shape, extent, and relative position of individual digit representations. Our results demonstrate the presence of very stable fine-grain somatotopy of the digits in human S1 and raise the issue of population variability in such detailed functional architecture of the human brain. These findings have implications for the study of detailed sensorimotor plasticity in the context of both learning and pathological dysfunction. The simple task and 10 min scan required to derive these maps also raises the potential for this paradigm as a tool in the clinical setting.
Significance statement: We applied ultra-high-resolution fMRI at 7 T to map sensory digit representations in the human primary somatosensory cortex (S1) at the level of individual participants across multiple time points. The resulting fine-grain maps of individual digits in S1 reveal the stability in this fine-grain functional organization over time, contrasted with the variability in these maps across individuals.
Keywords: 7 T; digit; mapping; reproducibility; somatotopy.
Copyright © 2016 Kolasinski et al.
Figures


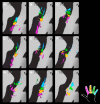
References
-
- Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol. 1991;66:1048–1058. - PubMed
-
- Baumgartner C, Doppelbauer A, Deecke L, Barth DS, Zeitlhofer J, Lindinger G, Sutherling WW. Neuromagnetic investigation of somatotopy of human hand somatosensory cortex. Exp Brain Res. 1991;87:641–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
