The Dynamic Multisensory Engram: Neural Circuitry Underlying Crossmodal Object Recognition in Rats Changes with the Nature of Object Experience
- PMID: 26818515
- PMCID: PMC6604816
- DOI: 10.1523/JNEUROSCI.3043-15.2016
The Dynamic Multisensory Engram: Neural Circuitry Underlying Crossmodal Object Recognition in Rats Changes with the Nature of Object Experience
Abstract
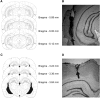
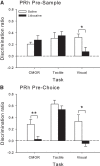
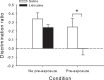
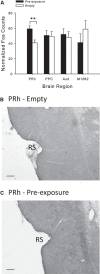
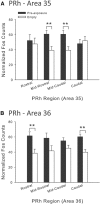
Rats, humans, and monkeys demonstrate robust crossmodal object recognition (CMOR), identifying objects across sensory modalities. We have shown that rats' performance of a spontaneous tactile-to-visual CMOR task requires functional integration of perirhinal (PRh) and posterior parietal (PPC) cortices, which seemingly provide visual and tactile object feature processing, respectively. However, research with primates has suggested that PRh is sufficient for multisensory object representation. We tested this hypothesis in rats using a modification of the CMOR task in which multimodal preexposure to the to-be-remembered objects significantly facilitates performance. In the original CMOR task, with no preexposure, reversible lesions of PRh or PPC produced patterns of impairment consistent with modality-specific contributions. Conversely, in the CMOR task with preexposure, PPC lesions had no effect, whereas PRh involvement was robust, proving necessary for phases of the task that did not require PRh activity when rats did not have preexposure; this pattern was supported by results from c-fos imaging. We suggest that multimodal preexposure alters the circuitry responsible for object recognition, in this case obviating the need for PPC contributions and expanding PRh involvement, consistent with the polymodal nature of PRh connections and results from primates indicating a key role for PRh in multisensory object representation. These findings have significant implications for our understanding of multisensory information processing, suggesting that the nature of an individual's past experience with an object strongly determines the brain circuitry involved in representing that object's multisensory features in memory.
Significance statement: The ability to integrate information from multiple sensory modalities is crucial to the survival of organisms living in complex environments. Appropriate responses to behaviorally relevant objects are informed by integration of multisensory object features. We used crossmodal object recognition tasks in rats to study the neurobiological basis of multisensory object representation. When rats had no prior exposure to the to-be-remembered objects, the spontaneous ability to recognize objects across sensory modalities relied on functional interaction between multiple cortical regions. However, prior multisensory exploration of the task-relevant objects remapped cortical contributions, negating the involvement of one region and significantly expanding the role of another. This finding emphasizes the dynamic nature of cortical representation of objects in relation to past experience.
Keywords: c-fos; cross-modal; multisensory integration; parietal cortex; perirhinal cortex.
Copyright © 2016 the authors 0270-6474/16/361273-17$15.00/0.
Figures




Similar articles
-
A distributed cortical representation underlies crossmodal object recognition in rats.J Neurosci. 2010 May 5;30(18):6253-61. doi: 10.1523/JNEUROSCI.6073-09.2010. J Neurosci. 2010. PMID: 20445051 Free PMC article.
-
Crossmodal object recognition in rats with and without multimodal object pre-exposure: no effect of hippocampal lesions.Neurobiol Learn Mem. 2012 Oct;98(3):311-9. doi: 10.1016/j.nlm.2012.09.001. Epub 2012 Sep 10. Neurobiol Learn Mem. 2012. PMID: 22975081
-
Evidence for a specific role for muscarinic receptors in crossmodal object recognition in rats.Neurobiol Learn Mem. 2015 Feb;118:125-32. doi: 10.1016/j.nlm.2014.11.017. Epub 2014 Dec 6. Neurobiol Learn Mem. 2015. PMID: 25490059
-
Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval.Neurosci Biobehav Rev. 2008 Jul;32(5):1055-70. doi: 10.1016/j.neubiorev.2008.04.004. Epub 2008 Apr 24. Neurosci Biobehav Rev. 2008. PMID: 18499253 Review.
-
Functional imaging of human crossmodal identification and object recognition.Exp Brain Res. 2005 Oct;166(3-4):559-71. doi: 10.1007/s00221-005-2396-5. Epub 2005 Jul 19. Exp Brain Res. 2005. PMID: 16028028 Review.
Cited by
-
The medial prefrontal cortex - hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: Behavioral, anatomical and neurochemical properties.Neurosci Biobehav Rev. 2020 Jun;113:373-407. doi: 10.1016/j.neubiorev.2020.04.007. Epub 2020 Apr 13. Neurosci Biobehav Rev. 2020. PMID: 32298711 Free PMC article. Review.
-
Subpopulations of neurons in the perirhinal cortex enable both modality-specific and modality-invariant recognition of objects.PLoS Biol. 2024 Jun 26;22(6):e3002713. doi: 10.1371/journal.pbio.3002713. eCollection 2024 Jun. PLoS Biol. 2024. PMID: 38924050 Free PMC article.
-
Auditory-induced visual illusions in rodents measured by spontaneous behavioural response.Sci Rep. 2019 Dec 16;9(1):19211. doi: 10.1038/s41598-019-55664-z. Sci Rep. 2019. PMID: 31844094 Free PMC article.
-
Neuronal circuitry for recognition memory of object and place in rodent models.Neurosci Biobehav Rev. 2022 Oct;141:104855. doi: 10.1016/j.neubiorev.2022.104855. Epub 2022 Sep 8. Neurosci Biobehav Rev. 2022. PMID: 36089106 Free PMC article. Review.
-
A Novel Method for Training Mice in Visuo-Tactile 3-D Object Discrimination and Recognition.Front Behav Neurosci. 2018 Nov 13;12:274. doi: 10.3389/fnbeh.2018.00274. eCollection 2018. Front Behav Neurosci. 2018. PMID: 30555307 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
