Neuropeptide Y Impairs Retrieval of Extinguished Fear and Modulates Excitability of Neurons in the Infralimbic Prefrontal Cortex
- PMID: 26818517
- PMCID: PMC6604823
- DOI: 10.1523/JNEUROSCI.4955-13.2016
Neuropeptide Y Impairs Retrieval of Extinguished Fear and Modulates Excitability of Neurons in the Infralimbic Prefrontal Cortex
Abstract
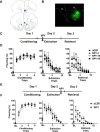
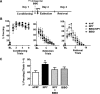
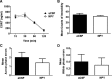
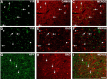
Neuropeptide Y (NPY), a 36 aa peptide, regulates stress and emotional behaviors. Preclinical and clinical studies support an association of NPY with trauma-evoked syndromes such as posttraumatic stress disorder (PTSD), although the exact contribution of NPY is not clear. In the current study, we examined functional attributes of NPY in the infralimbic (IL) cortex, an area that regulates fear memories and is reported to be hypoactive in PTSD. Carriers of NPY gene polymorphism rs16147 have been reported to have elevated prefrontal NPY expression. Infusion of NPY into the IL cortex in rats significantly impaired fear extinction memory without affecting conditioned fear expression or acquisition of extinction. Neuroendocrine stress response, depression-like behavior, and working memory performance were not affected by NPY infusion into the IL. The NPY Y1 receptor antagonist BIBO3304 completely abolished NPY effects on fear extinction retrieval. Y1 receptor expression was localized on CaMKII-positive pyramidal projection neurons and GAD67-positive interneurons in the IL. Patch-clamp recordings revealed increased inhibitory synaptic transmission onto IL projection neurons in the presence of NPY. Thus, NPY dampens excitability of IL projection neurons and impairs retrieval of extinction memory by inhibiting consolidation of extinction. Of relevance to PTSD, elevation of prefrontal NPY attributable to the genetic polymorphism rs16147 may contribute to IL hypoactivity, resulting in impaired extinction memory and susceptibility to the disorder.
Significance statement: Neuropeptide Y (NPY), a stress modulatory transmitter, is associated with posttraumatic stress disorder (PTSD). Contribution of NPY to PTSD symptomology is unclear. PTSD patients have reduced activity in the infralimbic (IL) subdivision of the medial prefrontal cortex (mPFC), associated with compromised extinction memory. No information exists on fear modulation by NPY in the IL cortex, although NPY and NPY receptors are abundant in these areas. This study shows that IL NPY inhibits consolidation of extinction, resulting in impaired retrieval of extinction memory and modulates excitability of IL projection neurons. In addition to providing a novel perspective on extinction memory modulation by NPY, our findings suggest that elevated mPFC NPY in gene polymorphism rs16147 carriers or after chronic stress could increase susceptibility to PTSD.
Keywords: Neuropeptide Y; Y1; extinction; fear; infralimbic; prefrontal.
Copyright © 2016 the authors 0270-6474/16/361306-10$15.00/0.
Figures

Similar articles
-
Trace Fear Conditioning Differentially Modulates Intrinsic Excitability of Medial Prefrontal Cortex-Basolateral Complex of Amygdala Projection Neurons in Infralimbic and Prelimbic Cortices.J Neurosci. 2015 Sep 30;35(39):13511-24. doi: 10.1523/JNEUROSCI.2329-15.2015. J Neurosci. 2015. PMID: 26424895 Free PMC article.
-
Role of NPY Y1 receptor on acquisition, consolidation and extinction on contextual fear conditioning: dissociation between anxiety, locomotion and non-emotional memory behavior.Neurobiol Learn Mem. 2013 Jul;103:26-33. doi: 10.1016/j.nlm.2013.04.005. Epub 2013 Apr 18. Neurobiol Learn Mem. 2013. PMID: 23603424
-
Neuropeptide Y reduces expression of social fear via simultaneous activation of Y1 and Y2 receptors.J Psychopharmacol. 2019 Dec;33(12):1533-1539. doi: 10.1177/0269881119862529. Epub 2019 Jul 22. J Psychopharmacol. 2019. PMID: 31328614 Free PMC article.
-
The role of Neuropeptide Y in fear conditioning and extinction.Neuropeptides. 2016 Feb;55:111-26. doi: 10.1016/j.npep.2015.09.007. Epub 2015 Sep 25. Neuropeptides. 2016. PMID: 26444585 Review.
-
Neuropeptide Y and posttraumatic stress disorder.Mol Psychiatry. 2013 Jun;18(6):646-55. doi: 10.1038/mp.2012.101. Epub 2012 Jul 17. Mol Psychiatry. 2013. PMID: 22801411 Free PMC article. Review.
Cited by
-
Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications.Mol Psychiatry. 2022 Jan;27(1):445-465. doi: 10.1038/s41380-021-01092-3. Epub 2021 Apr 19. Mol Psychiatry. 2022. PMID: 33875802 Free PMC article. Review.
-
Impaired Fear Extinction Recall in Serotonin Transporter Knockout Rats Is Transiently Alleviated during Adolescence.Brain Sci. 2019 May 22;9(5):118. doi: 10.3390/brainsci9050118. Brain Sci. 2019. PMID: 31121975 Free PMC article.
-
The Role of the Hippocampus in the Neuroendocrine Response to Neurobiological Stimuli in Experiment.Bull Exp Biol Med. 2021 Aug;171(4):494-498. doi: 10.1007/s10517-021-05258-5. Epub 2021 Sep 20. Bull Exp Biol Med. 2021. PMID: 34542755
-
Infralimbic cortical glutamate output is necessary for the neural and behavioral consequences of chronic stress.Neurobiol Stress. 2020 Nov 23;13:100274. doi: 10.1016/j.ynstr.2020.100274. eCollection 2020 Nov. Neurobiol Stress. 2020. PMID: 33344727 Free PMC article.
-
Infralimbic cortex controls fear memory generalization and susceptibility to extinction during consolidation.Sci Rep. 2020 Sep 28;10(1):15827. doi: 10.1038/s41598-020-72856-0. Sci Rep. 2020. PMID: 32985565 Free PMC article.
References
-
- Alho H, Ferrarese C, Vicini S, Vaccarino F. Subsets of GABAergic neurons in dissociated cell cultures of neonatal rat cerebral cortex show co-localization with specific modulator peptides. Brain Res. 1988;467:193–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
