Platelet-Activating Factor Receptors Mediate Excitatory Postsynaptic Hippocampal Injury in Experimental Autoimmune Encephalomyelitis
- PMID: 26818520
- PMCID: PMC4728729
- DOI: 10.1523/JNEUROSCI.1171-15.2016
Platelet-Activating Factor Receptors Mediate Excitatory Postsynaptic Hippocampal Injury in Experimental Autoimmune Encephalomyelitis
Abstract
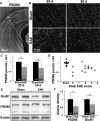
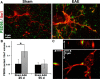
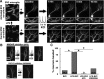
Gray matter degeneration contributes to progressive disability in multiple sclerosis (MS) and can occur out of proportion to measures of white matter disease. Although white matter pathology, including demyelination and axon injury, can lead to secondary gray matter changes, we hypothesized that neurons can undergo direct excitatory injury within the gray matter independent of these. We tested this using a model of experimental autoimmune encephalomyelitis (EAE) with hippocampal degeneration in C57BL/6 mice, in which immunofluorescent staining showed a 28% loss of PSD95-positive excitatory postsynaptic puncta in hippocampal area CA1 compared with sham-immunized controls, despite preservation of myelin and VGLUT1-positive excitatory axon terminals. Loss of postsynaptic structures was accompanied by appearance of PSD95-positive debris that colocalized with the processes of activated microglia at 25 d after immunization, and clearance of debris was followed by persistently reduced synaptic density at 55 d. In vitro, addition of activated BV2 microglial cells to hippocampal cultures increased neuronal vulnerability to excitotoxic dendritic damage following a burst of synaptic activity in a manner dependent on platelet-activating factor receptor (PAFR) signaling. In vivo treatment with PAFR antagonist BN52021 prevented PSD95-positive synapse loss in hippocampi of mice with EAE but did not affect development of EAE or local microglial activation. These results demonstrate that postsynaptic structures can be a primary target of injury within the gray matter in autoimmune neuroinflammatory disease, and suggest that this may occur via PAFR-mediated modulation of activity-dependent synaptic physiology downstream of microglial activation.
Significance statement: Unraveling gray matter degeneration is critical for developing treatments for progressive disability and cognitive impairment in multiple sclerosis (MS). In a mouse model of MS, we show that neurons can undergo injury at their synaptic connections within the gray matter, independent of the white matter pathology, demyelination, and axon injury that have been the focus of most current and emerging treatments. Damage to excitatory synapses in the hippocampus occurs in association with activated microglia, which can promote excitotoxic injury via activation of receptors for platelet-activating factor, a proinflammatory signaling molecule elevated in the brain in MS. Platelet-activating factor receptor blockade protected synapses in the mouse model, identifying a potential target for neuroprotective treatments in MS.
Keywords: excitotoxicity; microglia; multiple sclerosis; neurodegeneration; neuroprotection; platelet-activating factor.
Copyright © 2016 the authors 0270-6474/16/361336-11$15.00/0.
Figures




References
-
- Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D'Amelio M, Cavallucci V, Martorana A, Bergamaschi A, Cencioni MT, Diamantini A, Butti E, Comi G, Bernardi G, Cecconi F, Battistini L, Furlan R, Martino G. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29:3442–3452. doi: 10.1523/JNEUROSCI.5804-08.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
