Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain
- PMID: 26818526
- PMCID: PMC6604825
- DOI: 10.1523/JNEUROSCI.2456-15.2016
Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain
Abstract
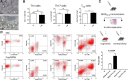
Brain-intrinsic degenerative cascades have been proposed to be an initial factor driving lesion formation in multiple sclerosis (MS). Here, we identify neurodegeneration as a potent trigger for peripheral immune cell recruitment into the mouse forebrain. Female C57BL/6 mice were fed cuprizone for 3 weeks, followed by a period of 2 weeks on normal chow to induce the formation of lesion foci in the forebrain. Subsequent immunization with myelin oligodendrocyte glycoprotein 35-55 peptide, which induces myelin autoreactive T cells in the periphery, resulted in massive immune cell recruitment into the affected forebrain. Additional adoptive transfer experiments together with flow cytometry analysis underline the importance of brain-derived signals for immune cell recruitment. This study clearly illustrates the significance of brain-intrinsic degenerative cascades for immune cell recruitment and MS lesion formation. Additional studies have to address the signaling cascades and mechanistic processes that form the top-down communication between the affected brain area, neurovascular unit, and peripheral immune cells.
Significance statement: We identify neurodegeneration as a potent trigger for peripheral immune cell recruitment into the forebrain. Thus, immune cell recruitment might be a second step during the formation of new inflammatory lesions in multiple sclerosis. A better understanding of factors regulating neurodegeneration-induced immune cell recruitment will pave the way for the development of novel therapeutic treatment strategies.
Keywords: cytodegeneration; invasion; trigger.
Copyright © 2016 the authors 0270-6474/16/361410-06$15.00/0.
Figures

References
-
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Brück W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. - PubMed
-
- Baxi EG, DeBruin J, Tosi DM, Grishkan IV, Smith MD, Kirby LA, Strasburger HJ, Fairchild AN, Calabresi PA, Gocke AR. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J Neurosci. 2015;35:8626–8639. doi: 10.1523/JNEUROSCI.3817-14.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
