Functional Topography of Human Auditory Cortex
- PMID: 26818527
- PMCID: PMC4728734
- DOI: 10.1523/JNEUROSCI.0226-15.2016
Functional Topography of Human Auditory Cortex
Abstract
Functional and anatomical studies have clearly demonstrated that auditory cortex is populated by multiple subfields. However, functional characterization of those fields has been largely the domain of animal electrophysiology, limiting the extent to which human and animal research can inform each other. In this study, we used high-resolution functional magnetic resonance imaging to characterize human auditory cortical subfields using a variety of low-level acoustic features in the spectral and temporal domains. Specifically, we show that topographic gradients of frequency preference, or tonotopy, extend along two axes in human auditory cortex, thus reconciling historical accounts of a tonotopic axis oriented medial to lateral along Heschl's gyrus and more recent findings emphasizing tonotopic organization along the anterior-posterior axis. Contradictory findings regarding topographic organization according to temporal modulation rate in acoustic stimuli, or "periodotopy," are also addressed. Although isolated subregions show a preference for high rates of amplitude-modulated white noise (AMWN) in our data, large-scale "periodotopic" organization was not found. Organization by AM rate was correlated with dominant pitch percepts in AMWN in many regions. In short, our data expose early auditory cortex chiefly as a frequency analyzer, and spectral frequency, as imposed by the sensory receptor surface in the cochlea, seems to be the dominant feature governing large-scale topographic organization across human auditory cortex.
Significance statement: In this study, we examine the nature of topographic organization in human auditory cortex with fMRI. Topographic organization by spectral frequency (tonotopy) extended in two directions: medial to lateral, consistent with early neuroimaging studies, and anterior to posterior, consistent with more recent reports. Large-scale organization by rates of temporal modulation (periodotopy) was correlated with confounding spectral content of amplitude-modulated white-noise stimuli. Together, our results suggest that the organization of human auditory cortex is driven primarily by its response to spectral acoustic features, and large-scale periodotopy spanning across multiple regions is not supported. This fundamental information regarding the functional organization of early auditory cortex will inform our growing understanding of speech perception and the processing of other complex sounds.
Keywords: auditory cortex; fMRI; tonotopy.
Copyright © 2016 the authors 0270-6474/16/361416-13$15.00/0.
Figures

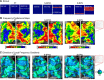

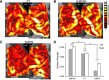
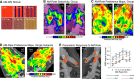
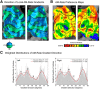


Similar articles
-
Distinct Representations of Tonotopy and Pitch in Human Auditory Cortex.J Neurosci. 2022 Jan 19;42(3):416-434. doi: 10.1523/JNEUROSCI.0960-21.2021. Epub 2021 Nov 19. J Neurosci. 2022. PMID: 34799415 Free PMC article.
-
Processing of spectral and amplitude envelope of animal vocalizations in the human auditory cortex.Neuropsychologia. 2010 Aug;48(10):2824-32. doi: 10.1016/j.neuropsychologia.2010.05.024. Epub 2010 May 21. Neuropsychologia. 2010. PMID: 20493891
-
Spatial representations of temporal and spectral sound cues in human auditory cortex.Cortex. 2013 Nov-Dec;49(10):2822-33. doi: 10.1016/j.cortex.2013.04.003. Epub 2013 Apr 24. Cortex. 2013. PMID: 23706955
-
Stimulus-dependent activations and attention-related modulations in the auditory cortex: a meta-analysis of fMRI studies.Hear Res. 2014 Jan;307:29-41. doi: 10.1016/j.heares.2013.08.001. Epub 2013 Aug 11. Hear Res. 2014. PMID: 23938208 Review.
-
Segmental processing in the human auditory dorsal stream.Brain Res. 2008 Jul 18;1220:179-90. doi: 10.1016/j.brainres.2007.11.013. Epub 2007 Nov 17. Brain Res. 2008. PMID: 18096139 Review.
Cited by
-
Joint Representation of Spatial and Phonetic Features in the Human Core Auditory Cortex.Cell Rep. 2018 Aug 21;24(8):2051-2062.e2. doi: 10.1016/j.celrep.2018.07.076. Cell Rep. 2018. PMID: 30134167 Free PMC article.
-
The Representation of Time Windows in Primate Auditory Cortex.Cereb Cortex. 2022 Aug 3;32(16):3568-3580. doi: 10.1093/cercor/bhab434. Cereb Cortex. 2022. PMID: 34875029 Free PMC article.
-
Topological Maps and Brain Computations From Low to High.Front Syst Neurosci. 2022 May 27;16:787737. doi: 10.3389/fnsys.2022.787737. eCollection 2022. Front Syst Neurosci. 2022. PMID: 35747394 Free PMC article.
-
Explaining event-related fields by a mechanistic model encapsulating the anatomical structure of auditory cortex.Biol Cybern. 2019 Jun;113(3):321-345. doi: 10.1007/s00422-019-00795-9. Epub 2019 Feb 28. Biol Cybern. 2019. PMID: 30820663 Free PMC article.
-
Is Human Auditory Cortex Organization Compatible With the Monkey Model? Contrary Evidence From Ultra-High-Field Functional and Structural MRI.Cereb Cortex. 2019 Jan 1;29(1):410-428. doi: 10.1093/cercor/bhy267. Cereb Cortex. 2019. PMID: 30357410 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
