Xuefu Zhuyu decoction, a traditional Chinese medicine, provides neuroprotection in a rat model of traumatic brain injury via an anti-inflammatory pathway
- PMID: 26818584
- PMCID: PMC4730240
- DOI: 10.1038/srep20040
Xuefu Zhuyu decoction, a traditional Chinese medicine, provides neuroprotection in a rat model of traumatic brain injury via an anti-inflammatory pathway
Abstract
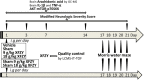
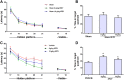
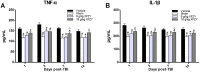
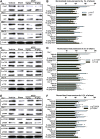
Neuroinflammation is central to the pathology of traumatic brain injury (TBI). Xuefu Zhuyu decoction (XFZY) is an effective traditional Chinese medicine to treat TBI. To elucidate its potential molecular mechanism, this study aimed to demonstrate that XFZY functions as an anti-inflammatory agent by inhibiting the PI3K-AKT-mTOR pathway. Sprague-Dawley rats were exposed to controlled cortical impact to produce a neuroinflammatory response. The treatment groups received XFZY (9 g/kg and 18 g/kg), Vehicle group and Sham group were gavaged with equal volumes of saline. The modified neurologic severity score (mNSS) and the Morris water maze test were used to assess neurological deficits. Arachidonic acid (AA) levels in brain tissue were measured using tandem gas chromatography-mass spectrometry. TNF-α and IL-1β levels in injured ipsilateral brain tissue were detected by ELISA. AKT and mTOR expression were measured by western blot analysis. The results indicated that XFZY significantly enhanced spatial memory acquisition. XFZY (especially at a dose of 9 g/kg) markedly reduced the mNSS and levels of AA, TNF-α and IL-1β. Significant downregulation of AKT/mTOR/p70S6K proteins in brain tissues was observed after the administration of XFZY (especially at a dose of 9 g/kg). XFZY may be a promising therapeutic strategy for reducing inflammation in TBI.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

