Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury
- PMID: 26818617
- PMCID: PMC5532463
- DOI: 10.1136/gutjnl-2015-310752
Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury
Erratum in
-
Correction: Signally via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury.Gut. 2020 Mar;69(3):e2. doi: 10.1136/gutjnl-2015-310752corr1. Gut. 2020. PMID: 32034032 Free PMC article. No abstract available.
Abstract
Objective: Liver fibrosis is associated with significant collagen-I deposition largely produced by activated hepatic stellate cells (HSCs); yet, the link between hepatocyte damage and the HSC profibrogenic response remains unclear. Here we show significant induction of osteopontin (OPN) and high-mobility group box-1 (HMGB1) in liver fibrosis. Since OPN was identified as upstream of HMGB1, we hypothesised that OPN could participate in the pathogenesis of liver fibrosis by increasing HMGB1 to upregulate collagen-I expression.
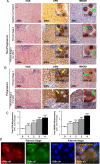
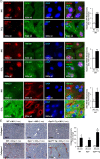
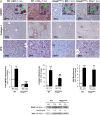
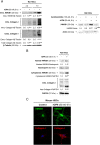
Design and results: Patients with long-term hepatitis C virus (HCV) progressing in disease stage displayed enhanced hepatic OPN and HMGB1 immunostaining, which correlated with fibrosis stage, whereas it remained similar in non-progressors. Hepatocyte cytoplasmic OPN and HMGB1 expression was significant while loss of nuclear HMGB1 occurred in patients with HCV-induced fibrosis compared with healthy explants. Well-established liver fibrosis along with marked induction of HMGB1 occurred in CCl4-injected OpnHep transgenic yet it was less in wild type and almost absent in Opn-/- mice. Hmgb1 ablation in hepatocytes (Hmgb1ΔHep) protected mice from CCl4-induced liver fibrosis. Coculture with hepatocytes that secrete OPN plus HMGB1 and challenge with recombinant OPN (rOPN) or HMGB1 (rHMGB1) enhanced collagen-I expression in HSCs, which was blunted by neutralising antibodies (Abs) and by Opn or Hmgb1 ablation. rOPN induced acetylation of HMGB1 in HSCs due to increased NADPH oxidase activity and the associated decrease in histone deacetylases 1/2 leading to upregulation of collagen-I. Last, rHMGB1 signalled via receptor for advanced glycation end-products and activated the PI3K-pAkt1/2/3 pathway to upregulate collagen-I.
Conclusions: During liver fibrosis, the increase in OPN induces HMGB1, which acts as a downstream alarmin driving collagen-I synthesis in HSCs.
Keywords: BASIC SCIENCES; HEPATIC FIBROSIS; HEPATIC STELLATE CELL; HEPATOCYTE; SIGNALING.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures







Comment in
-
Liver: Osteopontin and HMGB1: novel regulators of HSC activation.Nat Rev Gastroenterol Hepatol. 2016 Jun;13(6):320-2. doi: 10.1038/nrgastro.2016.58. Epub 2016 Apr 14. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27075262 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
