Safety and Efficacy of Granulocyte/Monocyte Apheresis in Steroid-Dependent Active Ulcerative Colitis with Insufficient Response or Intolerance to Immunosuppressants and/or Biologics [the ART Trial]: 12-week Interim Results
- PMID: 26818659
- PMCID: PMC4955912
- DOI: 10.1093/ecco-jcc/jjw032
Safety and Efficacy of Granulocyte/Monocyte Apheresis in Steroid-Dependent Active Ulcerative Colitis with Insufficient Response or Intolerance to Immunosuppressants and/or Biologics [the ART Trial]: 12-week Interim Results
Abstract
Background and aims: Patients with active, steroid-dependent ulcerative colitis with insufficient response or intolerance to immunosuppressants and/or biologic therapies have limited treatment options. Adacolumn, a granulocyte/monocyte adsorptive apheresis device, has shown clinical benefit in these patients. This study aimed to provide additional clinical data regarding the safety and efficacy of Adacolumn in this patient subgroup.
Methods: This single-arm, open-label, multicentre trial [ART] was conducted at 18 centres across the UK, France, and Germany. Eligible patients were 18-75 years old with moderate-to-severe, steroid-dependent active ulcerative colitis with insufficient response or intolerance to immunosuppressants and/or biologics. Patients received ≥ 5 weekly apheresis sessions with Adacolumn. The primary endpoint was clinical remission rate [clinical activity index ≤ 4] at Week 12.
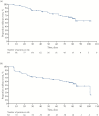
Results: In all, 86 patients were enrolled. At Week 12, 33/84 [39.3%] of patients in the intention-to-treat population achieved clinical remission, with 47/84 [56.0%] achieving a clinical response [clinical activity index reduction of ≥ 3]. Clinical remission was achieved in 30.0% of patients with previous immunosuppressant and biologic failure; steroid-free clinical remission and response were observed in 22.6% and 35.7% of these patients, respectively. Quality of life [Short Health Scale] significantly improved at Week 12 [p < 0.0001]. The majority of adverse events were of mild/moderate intensity.
Conclusions: At Week 12, Adacolumn provided significant clinical benefit in a large cohort of steroid-dependent ulcerative colitis patients with previous failure to immunosuppressant and/or biologic treatment, with a favourable safety profile. These results are consistent with previous studies and support Adacolumn use in this difficult-to-treat patient subgroup.
Keywords: Ulcerative colitis; adacolumn; apheresis.
Copyright © 2016 European Crohn’s and Colitis Organisation (ECCO). Published by Oxford University Press.
Figures

Similar articles
-
Granulocyte/monocyte adsorptive apheresis for the treatment of therapy-refractory chronic active ulcerative colitis.Scand J Gastroenterol. 2018 Apr;53(4):442-448. doi: 10.1080/00365521.2018.1447598. Epub 2018 Mar 7. Scand J Gastroenterol. 2018. PMID: 29513111 Clinical Trial.
-
In patients with ulcerative colitis, adsorptive depletion of granulocytes and monocytes impacts mucosal level of neutrophils and clinically is most effective in steroid naïve patients.Dig Liver Dis. 2008 Sep;40(9):731-6. doi: 10.1016/j.dld.2008.02.012. Epub 2008 Apr 2. Dig Liver Dis. 2008. PMID: 18387860
-
Treating ulcerative colitis by Adacolumn therapeutic leucocytapheresis: clinical efficacy and safety based on surveillance of 656 patients in 53 centres in Japan.Dig Liver Dis. 2009 Aug;41(8):570-7. doi: 10.1016/j.dld.2008.11.020. Epub 2009 Feb 10. Dig Liver Dis. 2009. PMID: 19211314
-
Safety and clinical efficacy of granulocyte and monocyte adsorptive apheresis therapy for ulcerative colitis.World J Gastroenterol. 2006 Jan 28;12(4):520-5. doi: 10.3748/wjg.v12.i4.520. World J Gastroenterol. 2006. PMID: 16489663 Free PMC article. Review.
-
Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes.Ther Apher Dial. 2003 Feb;7(1):48-59. doi: 10.1046/j.1526-0968.2003.00012.x. Ther Apher Dial. 2003. PMID: 12921115 Review.
Cited by
-
A multicenter retrospective study aiming to identify patients who respond well to adsorptive granulomonocytapheresis in moderately to severely active ulcerative colitis.Clin Transl Gastroenterol. 2018 Jul 6;9(7):170. doi: 10.1038/s41424-018-0037-0. Clin Transl Gastroenterol. 2018. PMID: 29977035 Free PMC article.
-
Efficacy of granulocyte and monocyte apheresis for antibiotic-refractory pouchitis after proctocolectomy for ulcerative colitis: an open-label, prospective, multicentre study.Therap Adv Gastroenterol. 2017 Feb;10(2):199-206. doi: 10.1177/1756283X16679348. Epub 2016 Nov 25. Therap Adv Gastroenterol. 2017. PMID: 28203278 Free PMC article.
-
Immunological Mechanisms of Adsorptive Cytapheresis in Inflammatory Bowel Disease.Dig Dis Sci. 2017 Jun;62(6):1417-1425. doi: 10.1007/s10620-017-4577-z. Epub 2017 Apr 21. Dig Dis Sci. 2017. PMID: 28432476 Review.
-
Faecal calprotectin level for assessing endoscopic activity and predicting future clinical course in patients with moderately active ulcerative colitis undergoing granulomonocytapheresis: a prospective cohort study.BMC Gastroenterol. 2018 Aug 1;18(1):120. doi: 10.1186/s12876-018-0853-4. BMC Gastroenterol. 2018. PMID: 30068300 Free PMC article.
-
Leukocytapheresis in patients with inflammatory bowel diseases.Prz Gastroenterol. 2021;6(2):99-105. doi: 10.5114/pg.2021.106658. Epub 2021 Jun 4. Prz Gastroenterol. 2021. PMID: 34276835 Free PMC article. Review.
References
-
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–25. - PubMed
-
- Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions and diagnosis. J Crohns Colitis 2012;6:965–90. - PubMed
-
- Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012;6:991–1030. - PubMed
-
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

