Cardiac expression of the CREM repressor isoform CREM-IbΔC-X in mice leads to arrhythmogenic alterations in ventricular cardiomyocytes
- PMID: 26818679
- PMCID: PMC4729809
- DOI: 10.1007/s00395-016-0532-y
Cardiac expression of the CREM repressor isoform CREM-IbΔC-X in mice leads to arrhythmogenic alterations in ventricular cardiomyocytes
Abstract
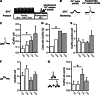
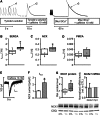
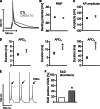
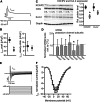
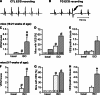
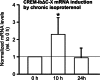
Chronic β-adrenergic stimulation is regarded as a pivotal step in the progression of heart failure which is associated with a high risk for arrhythmia. The cAMP-dependent transcription factors cAMP-responsive element binding protein (CREB) and cAMP-responsive element modulator (CREM) mediate transcriptional regulation in response to β-adrenergic stimulation and CREM repressor isoforms are induced after stimulation of the β-adrenoceptor. Here, we investigate whether CREM repressors contribute to the arrhythmogenic remodeling in the heart by analyzing arrhythmogenic alterations in ventricular cardiomyocytes (VCMs) from mice with transgenic expression of the CREM repressor isoform CREM-IbΔC-X (TG). Patch clamp analyses, calcium imaging, immunoblotting and real-time quantitative RT-PCR were conducted to study proarrhythmic alterations in TG VCMs vs. wild-type controls. The percentage of VCMs displaying spontaneous supra-threshold transient-like Ca(2+) releases was increased in TG accompanied by an enhanced transduction rate of sub-threshold Ca(2+) waves into these supra-threshold events. As a likely cause we discovered enhanced NCX-mediated Ca(2+) transport and NCX1 protein level in TG. An increase in I NCX and decrease in I to and its accessory channel subunit KChIP2 was associated with action potential prolongation and an increased proportion of TG VCMs showing early afterdepolarizations. Finally, ventricular extrasystoles were augmented in TG mice underlining the in vivo relevance of our findings. Transgenic expression of CREM-IbΔC-X in mouse VCMs leads to distinct arrhythmogenic alterations. Since CREM repressors are inducible by chronic β-adrenergic stimulation our results suggest that the inhibition of CRE-dependent transcription contributes to the formation of an arrhythmogenic substrate in chronic heart disease.
Keywords: Arrhythmia; NCX; Remodeling; Transcription factor CREM.
Figures


Similar articles
-
Inward Rectifier K+ Currents Contribute to the Proarrhythmic Electrical Phenotype of Atria Overexpressing Cyclic Adenosine Monophosphate Response Element Modulator Isoform CREM-IbΔC-X.J Am Heart Assoc. 2020 Dec;9(23):e016144. doi: 10.1161/JAHA.119.016144. Epub 2020 Nov 16. J Am Heart Assoc. 2020. PMID: 33191843 Free PMC article.
-
Characterization of the Genetic Program Linked to the Development of Atrial Fibrillation in CREM-IbΔC-X Mice.Circ Arrhythm Electrophysiol. 2017 Aug;10(8):e005075. doi: 10.1161/CIRCEP.117.005075. Circ Arrhythm Electrophysiol. 2017. PMID: 28784605
-
Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice.J Biol Chem. 2005 Feb 25;280(8):6906-14. doi: 10.1074/jbc.M407864200. Epub 2004 Nov 29. J Biol Chem. 2005. PMID: 15569686
-
Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM.Exp Cell Res. 2002 May 1;275(2):143-54. doi: 10.1006/excr.2002.5491. Exp Cell Res. 2002. PMID: 11969286 Review.
-
Coupling signalling pathways to transcriptional control: nuclear factors responsive to cAMP.Recent Prog Horm Res. 1997;52:121-39; discussion 139-40. Recent Prog Horm Res. 1997. PMID: 9238850 Review.
Cited by
-
Rolipram, a PDE4 Inhibitor, Enhances the Inotropic Effect of Rat Heart by Activating SERCA2a.Front Pharmacol. 2019 Mar 22;10:221. doi: 10.3389/fphar.2019.00221. eCollection 2019. Front Pharmacol. 2019. PMID: 30967774 Free PMC article.
-
Hypercontractile cardiac phenotype in mice overexpressing the regulatory subunit PR72 of protein phosphatase 2A.Front Cardiovasc Med. 2023 Oct 6;10:1239555. doi: 10.3389/fcvm.2023.1239555. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37868783 Free PMC article.
-
Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation.Circulation. 2018 Nov 13;138(20):2227-2242. doi: 10.1161/CIRCULATIONAHA.118.035202. Circulation. 2018. PMID: 29802206 Free PMC article.
-
Loss of protein phosphatase 2A regulatory subunit PPP2R5A is associated with increased incidence of stress-induced proarrhythmia.Front Cardiovasc Med. 2024 May 28;11:1419597. doi: 10.3389/fcvm.2024.1419597. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38863902 Free PMC article.
-
Impact of High Altitude on Cardiovascular Health: Current Perspectives.Vasc Health Risk Manag. 2021 Jun 8;17:317-335. doi: 10.2147/VHRM.S294121. eCollection 2021. Vasc Health Risk Manag. 2021. PMID: 34135590 Free PMC article. Review.
References
-
- Bai Y, Jones PP, Guo J, Zhong X, Clark RB, Zhou Q, Wang R, Vallmitjana A, Benitez R, Hove-Madsen L, Semeniuk L, Guo A, Song L, Duff HJ, Chen SRW. Phospholamban knockout breaks arrhythmogenic Ca2+ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice. Circ Res. 2013;113:517–526. doi: 10.1161/CIRCRESAHA.113.301678. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

