Complex interaction between dengue virus replication and expression of miRNA-133a
- PMID: 26818704
- PMCID: PMC4728791
- DOI: 10.1186/s12879-016-1364-y
Complex interaction between dengue virus replication and expression of miRNA-133a
Abstract
Background: Dengue virus (DENV) is the most common vector-borne viral infection worldwide with approximately 390 million cases and 25,000 reported deaths each year. MicroRNAs (miRNAs) are small non-coding RNA molecules responsible for the regulation of gene expression by repressing mRNA translation or inducing mRNA degradation. Although miRNAs possess antiviral activity against many mammalian-infecting viruses, their involvement in DENV replication is poorly understood.
Methods: Here, we explored the relationship between DENV and cellular microRNAs using bioinformatics tools. We overexpressed miRNA-133a in Vero cells to test its role in DENV replication and analyzed its expression using RT-qPCR. Furthermore, the expression of polypyrimidine tract binding protein (PTB), a protein involved in DENV replication, was analyzed by western blot. In addition, we profiled miRNA-133a expression in Vero cells challenged with DENV-2, using Taqman miRNA.
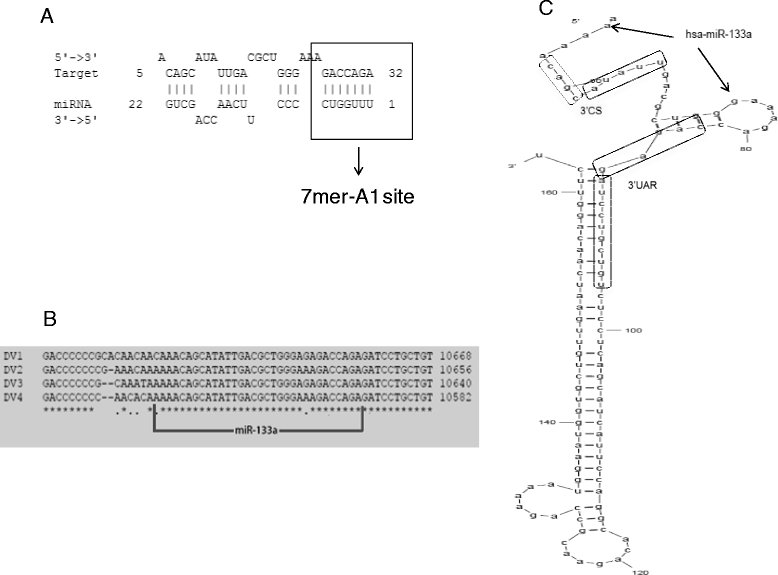
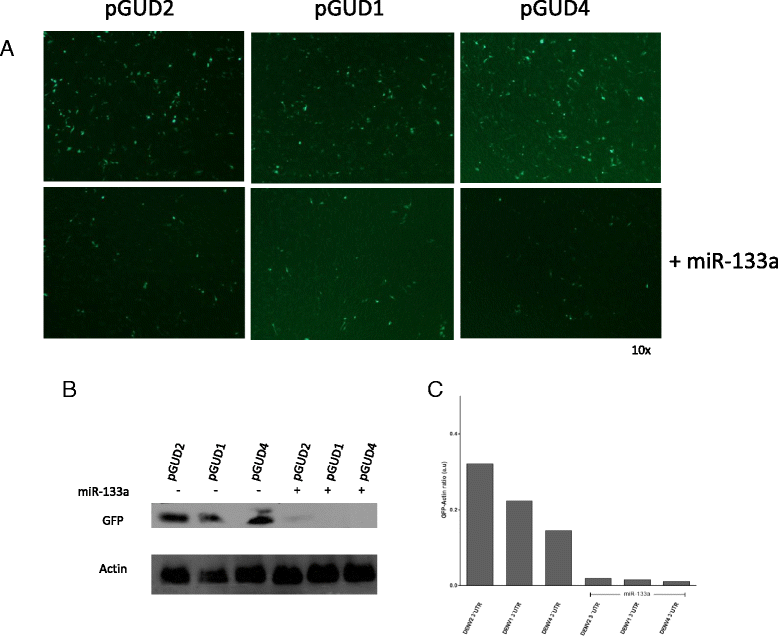
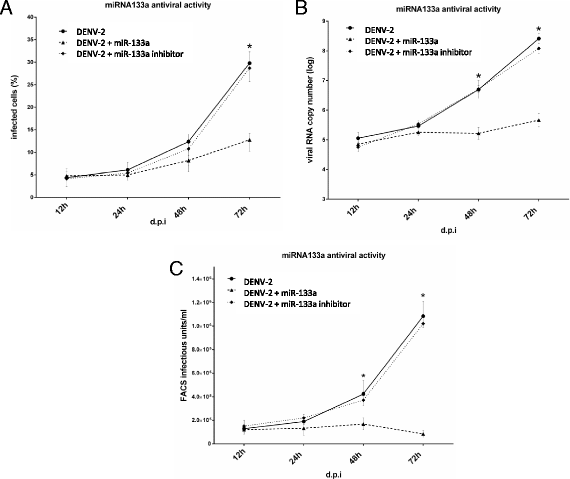
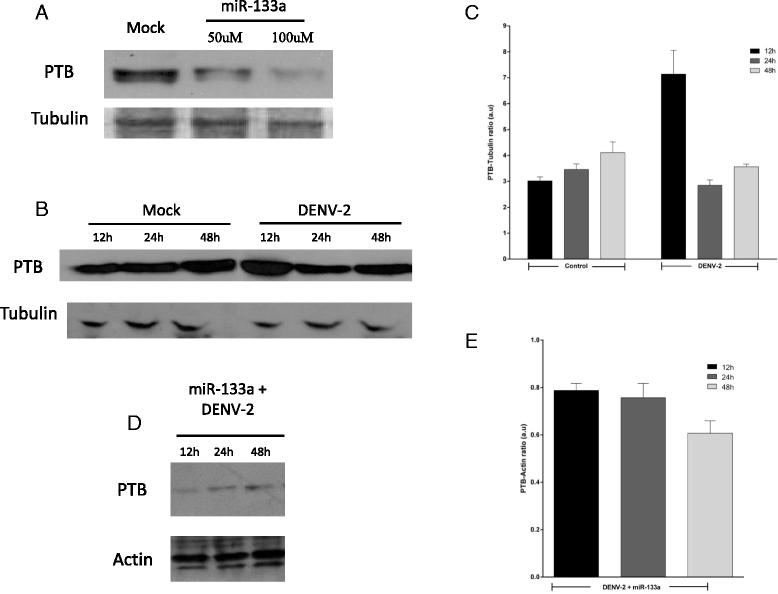
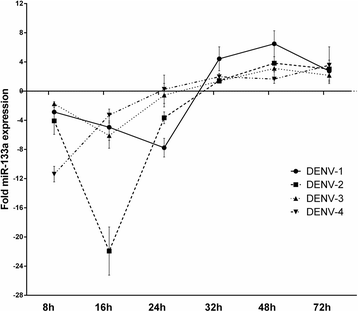
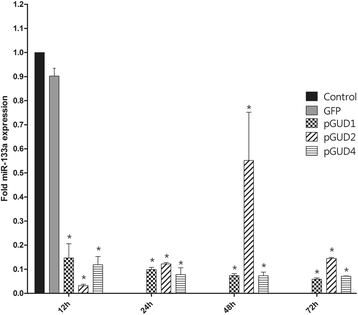
Results: Bioinformatic analysis revealed that the 3' untranslated region (3'UTR) of the DENV genome of all four DENV serotypes is targeted by several cellular miRNAs, including miRNA-133a. We found that overexpression of synthetic miRNA-133a suppressed DENV replication. Additionally, we observed that PTB transcription , a miRNA-133a target, is down-regulated during DENV infection. Based in our results we propose that 3'UTR of DENV down-regulates endogenous expression of miRNA-133a in Vero cells during the first hours of infection.
Conclusions: miRNA-133a regulates DENV replication possibly through the modulation of a host factor such as PTB. Further investigations are needed to verify whether miRNA-133a has an anti-DENV effect in vivo.
Figures
Similar articles
-
Overexpression of miR-484 and miR-744 in Vero cells alters Dengue virus replication.Mem Inst Oswaldo Cruz. 2017 Apr;112(4):281-291. doi: 10.1590/0074-02760160404. Epub 2017 Mar 2. Mem Inst Oswaldo Cruz. 2017. PMID: 28327787 Free PMC article.
-
Dynamic Interplay Between miR-133a and RBMX During Dengue Virus Infection.J Med Virol. 2025 Aug;97(8):e70543. doi: 10.1002/jmv.70543. J Med Virol. 2025. PMID: 40772600
-
Cellular microRNA-miR-548g-3p modulates the replication of dengue virus.J Infect. 2015 Jun;70(6):631-40. doi: 10.1016/j.jinf.2014.12.001. Epub 2014 Dec 11. J Infect. 2015. PMID: 25499200
-
Role of microRNAs in antiviral responses to dengue infection.J Biomed Sci. 2020 Jan 3;27(1):4. doi: 10.1186/s12929-019-0614-x. J Biomed Sci. 2020. PMID: 31898495 Free PMC article. Review.
-
Cross Talk between MicroRNAs and Dengue Virus.Am J Trop Med Hyg. 2024 Apr 2;110(5):856-867. doi: 10.4269/ajtmh.23-0546. Print 2024 May 1. Am J Trop Med Hyg. 2024. PMID: 38579704 Free PMC article. Review.
Cited by
-
Mammalian microRNA: an important modulator of host-pathogen interactions in human viral infections.J Biomed Sci. 2016 Oct 26;23(1):74. doi: 10.1186/s12929-016-0292-x. J Biomed Sci. 2016. PMID: 27784307 Free PMC article. Review.
-
Effect of miR-17 on Polygonum Cillinerve polysaccharide against transmissible gastroenteritis virus.Front Vet Sci. 2024 Feb 20;11:1360102. doi: 10.3389/fvets.2024.1360102. eCollection 2024. Front Vet Sci. 2024. PMID: 38444776 Free PMC article.
-
The Role of Noncoding RNA in the Transmission and Pathogenicity of Flaviviruses.Viruses. 2024 Feb 2;16(2):242. doi: 10.3390/v16020242. Viruses. 2024. PMID: 38400018 Free PMC article. Review.
-
A new host-targeted antiviral cyclolignan (SAU-22.107) for Dengue Virus infection in cell cultures. Potential action mechanisms based on cell imaging.Virus Res. 2023 Jan 2;323:198995. doi: 10.1016/j.virusres.2022.198995. Epub 2022 Nov 4. Virus Res. 2023. PMID: 36336130 Free PMC article.
-
Synergistic correlation between host angiogenin and dengue virus replication.RNA Biol. 2023 Jan;20(1):805-816. doi: 10.1080/15476286.2023.2264003. Epub 2023 Oct 5. RNA Biol. 2023. PMID: 37796112 Free PMC article.
References
-
- Dengue and dengue haemorrhagic fever. Fact sheet N.117 [http://www.who.int/mediacentre/factsheets/fs117/en/]
-
- WHO . Dengue: guidelines for diagnosis, treatment, prevention and control. In: Research WHOWatSPf, (TDR) aTiTD. France, editor. WHO Library Cataloguing-in-Publication Data. 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

