Serotonin neurons in the dorsal raphe nucleus encode reward signals
- PMID: 26818705
- PMCID: PMC4738365
- DOI: 10.1038/ncomms10503
Serotonin neurons in the dorsal raphe nucleus encode reward signals
Abstract
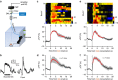
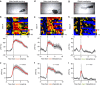
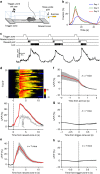
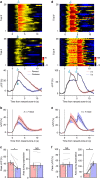
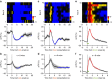
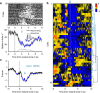
The dorsal raphe nucleus (DRN) is involved in organizing reward-related behaviours; however, it remains unclear how genetically defined neurons in the DRN of a freely behaving animal respond to various natural rewards. Here we addressed this question using fibre photometry and single-unit recording from serotonin (5-HT) neurons and GABA neurons in the DRN of behaving mice. Rewards including sucrose, food, sex and social interaction rapidly activate 5-HT neurons, but aversive stimuli including quinine and footshock do not. Both expected and unexpected rewards activate 5-HT neurons. After mice learn to wait for sucrose delivery, most 5-HT neurons fire tonically during waiting and then phasically on reward acquisition. Finally, GABA neurons are activated by aversive stimuli but inhibited when mice seek rewards. Thus, DRN 5-HT neurons positively encode a wide range of reward signals during anticipatory and consummatory phases of reward responses. Moreover, GABA neurons play a complementary role in reward processing.
Figures


References
-
- Schultz W., Dayan P. & Montague P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997). - PubMed
-
- Wise R. A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004). - PubMed
-
- Berridge K. C. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191, 391–431 (2007). - PubMed
-
- Jacobs B. L. & Azmitia E. C. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

