Cholinergic Targets in Lung Cancer
- PMID: 26818857
- PMCID: PMC4961355
- DOI: 10.2174/1381612822666160127114237
Cholinergic Targets in Lung Cancer
Abstract
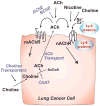
Lung cancers express an autocrine cholinergic loop in which secreted acetylcholine can stimulate tumor growth through both nicotinic and muscarinic receptors. Because activation of mAChR and nAChR stimulates growth; tumor growth can be stimulated by both locally synthesized acetylcholine as well as acetylcholine from distal sources and from nicotine in the high percentage of lung cancer patients who are smokers. The stimulation of lung cancer growth by cholinergic agonists offers many potential new targets for lung cancer therapy. Cholinergic signaling can be targeted at the level of choline transport; acetylcholine synthesis, secretion and degradation; and nicotinic and muscarinic receptors. In addition, the newly describe family of ly-6 allosteric modulators of nicotinic signaling such as lynx1 and lynx2 offers yet another new approach to novel lung cancer therapeutics. Each of these targets has their potential advantages and disadvantages for the development of new lung cancer therapies which are discussed in this review.
Figures




Similar articles
-
Acetylcholine signaling system in progression of lung cancers.Pharmacol Ther. 2019 Feb;194:222-254. doi: 10.1016/j.pharmthera.2018.10.002. Epub 2018 Oct 3. Pharmacol Ther. 2019. PMID: 30291908 Free PMC article. Review.
-
Activated cholinergic signaling provides a target in squamous cell lung carcinoma.Cancer Res. 2008 Jun 15;68(12):4693-700. doi: 10.1158/0008-5472.CAN-08-0183. Cancer Res. 2008. PMID: 18559515 Free PMC article.
-
Role of Lynx1 and related Ly6 proteins as modulators of cholinergic signaling in normal and neoplastic bronchial epithelium.Int Immunopharmacol. 2015 Nov;29(1):93-8. doi: 10.1016/j.intimp.2015.05.022. Epub 2015 May 26. Int Immunopharmacol. 2015. PMID: 26025503 Free PMC article.
-
Cholinergic-induced anion secretion in murine jejunal enteroids involves synergy between muscarinic and nicotinic pathways.Am J Physiol Cell Physiol. 2020 Aug 1;319(2):C321-C330. doi: 10.1152/ajpcell.00179.2020. Epub 2020 Jun 17. Am J Physiol Cell Physiol. 2020. PMID: 32551856 Free PMC article.
-
Neuronal Acetylcholine Nicotinic Receptors as New Targets for Lung Cancer Treatment.Curr Pharm Des. 2016;22(14):2160-9. doi: 10.2174/1381612822666160203144114. Curr Pharm Des. 2016. PMID: 26845123 Review.
Cited by
-
Acetylcholine signaling system in progression of lung cancers.Pharmacol Ther. 2019 Feb;194:222-254. doi: 10.1016/j.pharmthera.2018.10.002. Epub 2018 Oct 3. Pharmacol Ther. 2019. PMID: 30291908 Free PMC article. Review.
-
Muscarinic Receptors Associated with Cancer.Cancers (Basel). 2022 May 7;14(9):2322. doi: 10.3390/cancers14092322. Cancers (Basel). 2022. PMID: 35565451 Free PMC article. Review.
-
Potential immunologic and prognostic roles of CHRNA6 in SCLC and pan-cancer.Heliyon. 2024 Sep 26;10(19):e38572. doi: 10.1016/j.heliyon.2024.e38572. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39398083 Free PMC article.
-
Regulation of Cisplatin Resistance in Lung Cancer Cells by Nicotine, BDNF, and a β-Adrenergic Receptor Blocker.Int J Mol Sci. 2022 Oct 24;23(21):12829. doi: 10.3390/ijms232112829. Int J Mol Sci. 2022. PMID: 36361620 Free PMC article.
-
Orthosteric and/or Allosteric Binding of α-Conotoxins to Nicotinic Acetylcholine Receptors and Their Models.Mar Drugs. 2018 Nov 22;16(12):460. doi: 10.3390/md16120460. Mar Drugs. 2018. PMID: 30469507 Free PMC article.
References
-
- Wahbah M, Boroumand N, Castro C, El-Zeky F, Eltorky M. Changing trends in the distribution of the histologic types of lung cancer: a review of 4,439 cases. Ann Diagn Pathol. 2007;11:89–96. - PubMed
-
- Gabrielson E. Worldwide trends in lung cancer pathology. Respirology. 2006;11:533–8. - PubMed
-
- Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9. - PubMed
-
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Patholologic Basis of Disease. 8. Philadelphia, PA: W.B. Saunders Company; 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

