A unique self-organization of bacterial sub-communities creates iridescence in Cellulophaga lytica colony biofilms
- PMID: 26819100
- PMCID: PMC4730217
- DOI: 10.1038/srep19906
A unique self-organization of bacterial sub-communities creates iridescence in Cellulophaga lytica colony biofilms
Abstract
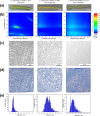
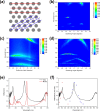
Iridescent color appearances are widespread in nature. They arise from the interaction of light with micron- and submicron-sized physical structures spatially arranged with periodic geometry and are usually associated with bright angle-dependent hues. Iridescence has been reported for many animals and marine organisms. However, iridescence has not been well studied in bacteria. Recently, we reported a brilliant "pointillistic" iridescence in colony biofilms of marine Flavobacteria that exhibit gliding motility. The mechanism of their iridescence is unknown. Here, using a multi-disciplinary approach, we show that the cause of iridescence is a unique periodicity of the cell population in the colony biofilm. Cells are arranged together to form hexagonal photonic crystals. Our model highlights a novel pattern of self-organization in a bacterial biofilm. "Pointillistic" bacterial iridescence can be considered a new light-dependent phenomenon for the field of microbiology.
Figures



Similar articles
-
Colony analysis and deep learning uncover 5-hydroxyindole as an inhibitor of gliding motility and iridescence in Cellulophaga lytica.Microbiology (Reading). 2018 Mar;164(3):308-321. doi: 10.1099/mic.0.000617. Epub 2018 Feb 5. Microbiology (Reading). 2018. PMID: 29458680
-
Effect of abiotic factors on the unique glitter-like iridescence of Cellulophaga lytica.FEMS Microbiol Lett. 2012 Aug;333(2):101-8. doi: 10.1111/j.1574-6968.2012.02614.x. Epub 2012 Jun 27. FEMS Microbiol Lett. 2012. PMID: 22681043
-
Iridescence of a marine bacterium and classification of prokaryotic structural colors.Appl Environ Microbiol. 2012 Apr;78(7):2092-9. doi: 10.1128/AEM.07339-11. Epub 2012 Jan 20. Appl Environ Microbiol. 2012. PMID: 22267664 Free PMC article.
-
The Paradox of Iridescent Signals.Trends Ecol Evol. 2021 Mar;36(3):187-195. doi: 10.1016/j.tree.2020.10.009. Epub 2020 Nov 6. Trends Ecol Evol. 2021. PMID: 33168152 Review.
-
Meat color and iridescence: Origin, analysis, and approaches to modulation.Compr Rev Food Sci Food Saf. 2023 Jul;22(4):3366-3394. doi: 10.1111/1541-4337.13191. Epub 2023 Jun 12. Compr Rev Food Sci Food Saf. 2023. PMID: 37306532 Review.
Cited by
-
Polysaccharide metabolism regulates structural colour in bacterial colonies.J R Soc Interface. 2022 May;19(190):20220181. doi: 10.1098/rsif.2022.0181. Epub 2022 May 25. J R Soc Interface. 2022. PMID: 35611622 Free PMC article.
-
On the colour of wing scales in butterflies: iridescence and preferred orientation of single gyroid photonic crystals.Interface Focus. 2017 Aug 6;7(4):20160154. doi: 10.1098/rsfs.2016.0154. Epub 2017 Jun 16. Interface Focus. 2017. PMID: 28630678 Free PMC article.
-
Intercellular adhesion promotes clonal mixing in growing bacterial populations.J R Soc Interface. 2018 Sep 19;15(146):20180406. doi: 10.1098/rsif.2018.0406. J R Soc Interface. 2018. PMID: 30232243 Free PMC article.
-
Does Structural Color Exist in True Fungi?J Fungi (Basel). 2021 Feb 16;7(2):141. doi: 10.3390/jof7020141. J Fungi (Basel). 2021. PMID: 33669274 Free PMC article.
-
Quantitative characterization of iridescent colours in biological studies: a novel method using optical theory.Interface Focus. 2019 Feb 6;9(1):20180049. doi: 10.1098/rsfs.2018.0049. Epub 2018 Dec 14. Interface Focus. 2019. PMID: 30603069 Free PMC article.
References
-
- Wiedenmann J., Oswald F. & Nienhaus G. U. Fluorescent proteins for live cell imaging: opportunities, limitations, and challenges. IUBMB Life 61, 1029–1042 (2009). - PubMed
-
- Widder E. A. Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science 328, 704–708 (2010). - PubMed
-
- Vukusic P. Materials science. Evolutionary photonics with a twist. Science 325, 398–399 (2009). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

