RNA sequencing-based analysis of the spleen transcriptome following infectious bronchitis virus infection of chickens selected for different mannose-binding lectin serum concentrations
- PMID: 26819139
- PMCID: PMC4729133
- DOI: 10.1186/s12864-016-2403-1
RNA sequencing-based analysis of the spleen transcriptome following infectious bronchitis virus infection of chickens selected for different mannose-binding lectin serum concentrations
Abstract
Background: Avian infectious bronchitis is a highly contagious disease of the upper-respiratory tract caused by infectious bronchitis virus (IBV). Understanding the molecular mechanisms involved in the interaction between innate and adaptive immune responses to IBV infection is a crucial element for further improvements in strategies to control IB. To this end, two chicken lines, selected for high (L10H line) and low (L10L line) serum concentration of mannose-binding lectin (MBL) were studied. In total, 32 birds from each line were used. Sixteen birds from each line were infected with IBV and sixteen were left uninfected. Eight uninfected and infected birds from each line were euthanized at 1 and 3 weeks post infection. RNA sequencing was performed on spleen samples from all 64 birds and differential gene expression analysis was performed for four comparisons: L10L line versus L10H line for uninfected birds at weeks 1 and 3, respectively, and in the same way for infected birds. Functional analysis was performed using Gene Ontology (GO) Immune System Process terms specific for Gallus gallus.
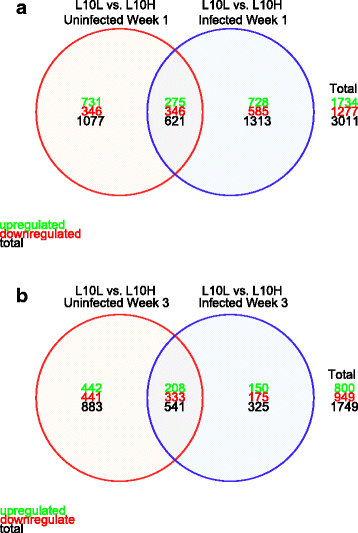
Results: Comparing uninfected L10H and L10L birds, we identified 1698 and 1424 differentially expressed (DE) genes at weeks 1 and 3, respectively. For the IBV-infected birds, 1934 and 866 DE genes were identified between the two lines at weeks 1 and 3, respectively. The two most enriched GO terms emerging from the comparison of uninfected birds between the two lines were "Lymphocyte activation involved in immune response" and "Somatic recombination of immunoglobulin genes involved in immune response" at weeks 1 and 3, respectively. When comparing IBV-infected birds between the two lines, the most enriched GO terms were "Alpha-beta T cell activation" and "Positive regulation of leukocyte activation" at weeks 1 and 3, respectively.
Conclusions: Healthy birds from the two lines showed significant differences in expression profiles for subsets of adaptive and innate immunity-related genes, whereas comparison of the IBV-infected birds from the two lines showed differences in expression of immunity-related genes involved in T cell activation and proliferation. The observed transcriptome differences between the two lines indicate that selection for MBL had influenced innate as well as adaptive immunity.
Figures



Similar articles
-
Characterization of cellular and humoral immune responses after IBV infection in chicken lines differing in MBL serum concentration.Viral Immunol. 2014 Dec;27(10):529-42. doi: 10.1089/vim.2014.0088. Viral Immunol. 2014. PMID: 25343382 Free PMC article.
-
Adjuvant effects of mannose-binding lectin ligands on the immune response to infectious bronchitis vaccine in chickens with high or low serum mannose-binding lectin concentrations.Immunobiology. 2014 Apr;219(4):263-74. doi: 10.1016/j.imbio.2013.10.013. Epub 2013 Nov 8. Immunobiology. 2014. PMID: 24305086 Free PMC article.
-
The consequence of low mannose-binding lectin plasma concentration in relation to susceptibility to Salmonella Infantis in chickens.Vet Immunol Immunopathol. 2015 Jan 15;163(1-2):23-32. doi: 10.1016/j.vetimm.2014.11.003. Epub 2014 Nov 20. Vet Immunol Immunopathol. 2015. PMID: 25487759
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
-
Avian infectious bronchitis virus.Rev Sci Tech. 2000 Aug;19(2):493-508. doi: 10.20506/rst.19.2.1228. Rev Sci Tech. 2000. PMID: 10935276 Review.
Cited by
-
Evaluation of viral load and transcriptome changes in tracheal tissue of two hybrids of commercial broiler chickens infected with avian infectious bronchitis virus: a comparative study.Arch Virol. 2022 Feb;167(2):377-391. doi: 10.1007/s00705-021-05322-5. Epub 2022 Jan 4. Arch Virol. 2022. PMID: 34981169 Free PMC article.
-
Host Immune Response Modulation in Avian Coronavirus Infection: Tracheal Transcriptome Profiling In Vitro and In Vivo.Viruses. 2024 Apr 14;16(4):605. doi: 10.3390/v16040605. Viruses. 2024. PMID: 38675946 Free PMC article.
-
Reference gene selection for gene expression study in shell gland and spleen of laying hens challenged with infectious bronchitis virus.Sci Rep. 2017 Oct 27;7(1):14271. doi: 10.1038/s41598-017-14693-2. Sci Rep. 2017. PMID: 29079779 Free PMC article.
-
Comparative transcriptome analysis reveals induction of apoptosis in chicken kidney cells associated with the virulence of nephropathogenic infectious bronchitis virus.Microb Pathog. 2017 Dec;113:451-459. doi: 10.1016/j.micpath.2017.11.031. Epub 2017 Nov 21. Microb Pathog. 2017. PMID: 29174688 Free PMC article.
-
GADD45β, an anti-tumor gene, inhibits avian leukosis virus subgroup J replication in chickens.Oncotarget. 2016 Oct 18;7(42):68883-68893. doi: 10.18632/oncotarget.12027. Oncotarget. 2016. PMID: 27655697 Free PMC article.
References
-
- Jackwood MW, de Wit S. Infectious Bronchitis. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair VL, editors. Diseases of Poultry. 13. Hoboken: Wiley-Blackwell; 2013. pp. 139–59.
-
- Abd El Rahman S, El-Kenawy AA, Neumann U, Herrler G, Winter C. Comparative analysis of the sialic acid binding activity and the tropism for the respiratory epithelium of four different strains of avian infectious bronchitis virus. Avian Pathol. 2009;38:41–5. doi: 10.1080/03079450802632049. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

