Type I Interferon response in olfactory bulb, the site of tick-borne flavivirus accumulation, is primarily regulated by IPS-1
- PMID: 26819220
- PMCID: PMC4730761
- DOI: 10.1186/s12974-016-0487-9
Type I Interferon response in olfactory bulb, the site of tick-borne flavivirus accumulation, is primarily regulated by IPS-1
Abstract
Background: Although type I interferons (IFNs)-key effectors of antiviral innate immunity are known to be induced via different pattern recognition receptors (PRRs), the cellular source and the relative contribution of different PRRs in host protection against viral infection is often unclear. IPS-1 is a downstream adaptor for retinoid-inducible gene I (RIG-I)-like receptor signaling. In this study, we investigate the relative contribution of IPS-1 in the innate immune response in the different brain regions during infection with tick-borne encephalitis virus (TBEV), a flavivirus that causes a variety of severe symptoms like hemorrhagic fevers, encephalitis, and meningitis in the human host.
Methods: IPS-1 knockout mice were infected with TBEV/Langat virus (LGTV), and viral burden in the peripheral and the central nervous systems, type I IFN induction, brain infiltrating cells, and inflammatory response was analyzed.
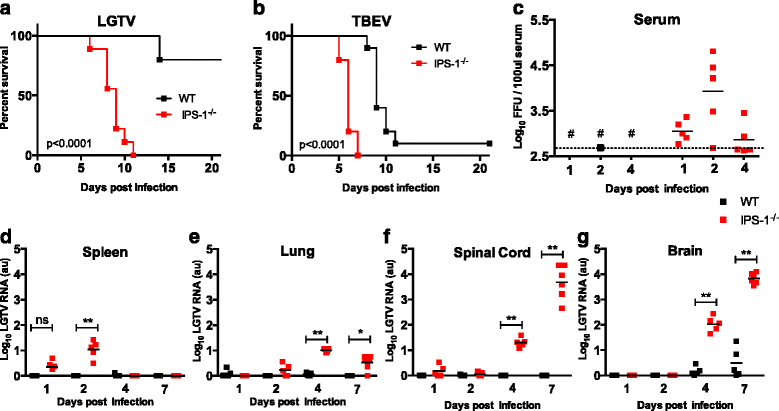
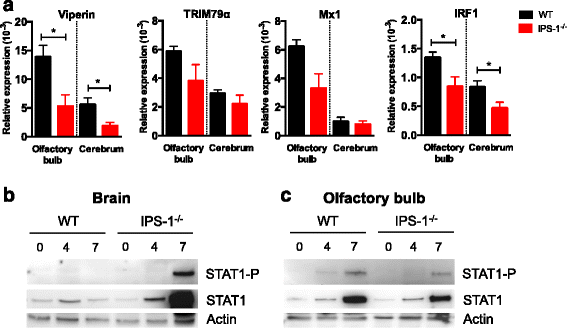
Results: We show that IPS-1 is indispensable for controlling TBEV and LGTV infections in the peripheral and central nervous system. Our data indicate that IPS-1 regulates neuropathogenicity in mice. IFN response is differentially regulated in distinct regions of the central nervous system (CNS) influencing viral tropism, as LGTV replication was mainly restricted to olfactory bulb in wild-type (WT) mice. In contrast to the other brain regions, IFN upregulation in the olfactory bulb was dependent on IPS-1 signaling. IPS-1 regulates basal levels of antiviral interferon-stimulated genes (ISGs) like viperin and IRF-1 which contributes to the establishment of early viral replication which inhibits STAT1 activation. This diminishes the antiviral response even in the presence of high IFN-β levels. Consequently, the absence of IPS-1 causes uncontrolled virus replication, in turn resulting in apoptosis, activation of microglia and astrocytes, elevated proinflammatory response, and recruitment of inflammatory cells into the CNS.
Conclusions: We show that LGTV replication is restricted to the olfactory bulb and that IPS-1 is a very important player in the olfactory bulb in shaping the innate immune response by inhibiting early viral replication and viral spread throughout the central nervous system. In the absence of IPS-1, higher viral replication leads to the evasion of antiviral response by inhibiting interferon signaling. Our data suggest that the local microenvironment of distinct brain regions is critical to determine virus permissiveness.
Figures




Similar articles
-
Cell-type- and region-specific restriction of neurotropic flavivirus infection by viperin.J Neuroinflammation. 2018 Mar 15;15(1):80. doi: 10.1186/s12974-018-1119-3. J Neuroinflammation. 2018. PMID: 29544502 Free PMC article.
-
Type I interferon protects mice from fatal neurotropic infection with Langat virus by systemic and local antiviral responses.J Virol. 2014 Nov;88(21):12202-12. doi: 10.1128/JVI.01215-14. Epub 2014 Aug 13. J Virol. 2014. PMID: 25122777 Free PMC article.
-
Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects.J Neuroinflammation. 2016 Oct 24;13(1):277. doi: 10.1186/s12974-016-0748-7. J Neuroinflammation. 2016. PMID: 27776548 Free PMC article.
-
Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis.Virus Res. 2005 Aug;111(2):161-74. doi: 10.1016/j.virusres.2005.04.007. Virus Res. 2005. PMID: 15871909 Review.
-
The host cell response to tick-borne encephalitis virus.Biochem Biophys Res Commun. 2017 Oct 28;492(4):533-540. doi: 10.1016/j.bbrc.2017.02.006. Epub 2017 Feb 4. Biochem Biophys Res Commun. 2017. PMID: 28167278 Review.
Cited by
-
MAVS signaling shapes microglia responses to neurotropic virus infection.J Neuroinflammation. 2024 Oct 18;21(1):264. doi: 10.1186/s12974-024-03258-6. J Neuroinflammation. 2024. PMID: 39425188 Free PMC article.
-
Secreted NS1 proteins of tick-borne encephalitis virus and West Nile virus block dendritic cell activation and effector functions.Microbiol Spectr. 2023 Sep 14;11(5):e0219223. doi: 10.1128/spectrum.02192-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37707204 Free PMC article.
-
IRE1-Mediated Unfolded Protein Response Promotes the Replication of Tick-Borne Flaviviruses in a Virus and Cell-Type Dependent Manner.Viruses. 2021 Oct 27;13(11):2164. doi: 10.3390/v13112164. Viruses. 2021. PMID: 34834970 Free PMC article.
-
A database of human genes and a gene network involved in response to tick-borne encephalitis virus infection.BMC Evol Biol. 2017 Dec 28;17(Suppl 2):259. doi: 10.1186/s12862-017-1107-8. BMC Evol Biol. 2017. PMID: 29297316 Free PMC article.
-
Astrocytes evoke a robust IRF7-independent type I interferon response upon neurotropic viral infection.J Neuroinflammation. 2023 Sep 22;20(1):213. doi: 10.1186/s12974-023-02892-w. J Neuroinflammation. 2023. PMID: 37737190 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

