Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports
- PMID: 26819231
- PMCID: PMC4729837
- DOI: 10.1136/bmj.i65
Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports
Abstract
Objective: To study serious harms associated with selective serotonin and serotonin-norepinephrine reuptake inhibitors.Design Systematic review and meta-analysis.
Main outcome measures: Mortality and suicidality. Secondary outcomes were aggressive behaviour and akathisia.
Data sources: Clinical study reports for duloxetine, fluoxetine, paroxetine, sertraline, and venlafaxine obtained from the European and UK drug regulators, and summary trial reports for duloxetine and fluoxetine from Eli Lilly's website.
Eligibility criteria for study selection: Double blind placebo controlled trials that contained any patient narratives or individual patient listings of harms.
Data extraction and analysis: Two researchers extracted data independently; the outcomes were meta-analysed by Peto's exact method (fixed effect model).
Results: We included 70 trials (64,381 pages of clinical study reports) with 18,526 patients. These trials had limitations in the study design and discrepancies in reporting, which may have led to serious under-reporting of harms. For example, some outcomes appeared only in individual patient listings in appendices, which we had for only 32 trials, and we did not have case report forms for any of the trials. Differences in mortality (all deaths were in adults, odds ratio 1.28, 95% confidence interval 0.40 to 4.06), suicidality (1.21, 0.84 to 1.74), and akathisia (2.04, 0.93 to 4.48) were not significant, whereas patients taking antidepressants displayed more aggressive behaviour (1.93, 1.26 to 2.95). For adults, the odds ratios were 0.81 (0.51 to 1.28) for suicidality, 1.09 (0.55 to 2.14) for aggression, and 2.00 (0.79 to 5.04) for akathisia. The corresponding values for children and adolescents were 2.39 (1.31 to 4.33), 2.79 (1.62 to 4.81), and 2.15 (0.48 to 9.65). In the summary trial reports on Eli Lilly's website, almost all deaths were noted, but all suicidal ideation events were missing, and the information on the remaining outcomes was incomplete.
Conclusions: Because of the shortcomings identified and having only partial access to appendices with no access to case report forms, the harms could not be estimated accurately. In adults there was no significant increase in all four outcomes, but in children and adolescents the risk of suicidality and aggression doubled. To elucidate the harms reliably, access to anonymised individual patient data is needed.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
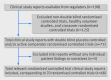

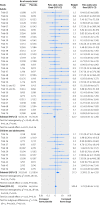

Comment in
-
Antidepressants may double risk of suicide and aggression in children, study finds.BMJ. 2016 Jan 28;352:i545. doi: 10.1136/bmj.i545. BMJ. 2016. PMID: 26821942 No abstract available.
-
Suicidality and aggression during antidepressant treatment: authors misinterpreted earlier paper from the FDA.BMJ. 2016 Feb 16;352:i906. doi: 10.1136/bmj.i906. BMJ. 2016. PMID: 26882915 No abstract available.
-
Paper on suicidality and aggression during antidepressant treatment was flawed and the press release was misleading.BMJ. 2016 Feb 16;352:i911. doi: 10.1136/bmj.i911. BMJ. 2016. PMID: 26883639 No abstract available.
-
Author's reply to Dubicka and colleagues and Stone.BMJ. 2016 Feb 16;352:i915. doi: 10.1136/bmj.i915. BMJ. 2016. PMID: 26884436 No abstract available.
-
Alcohol and serious harms of antidepressant treatment.BMJ. 2016 Feb 17;352:i892. doi: 10.1136/bmj.i892. BMJ. 2016. PMID: 26887780 No abstract available.
Similar articles
-
New generation antidepressants for depression in children and adolescents: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 24;5(5):CD013674. doi: 10.1002/14651858.CD013674.pub2. Cochrane Database Syst Rev. 2021. PMID: 34029378 Free PMC article.
-
Newer generation antidepressants for depressive disorders in children and adolescents.Cochrane Database Syst Rev. 2012 Nov 14;11(11):CD004851. doi: 10.1002/14651858.CD004851.pub3. Cochrane Database Syst Rev. 2012. PMID: 23152227 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article.
-
Vortioxetine for depression in adults.Cochrane Database Syst Rev. 2017 Jul 5;7(7):CD011520. doi: 10.1002/14651858.CD011520.pub2. Cochrane Database Syst Rev. 2017. PMID: 28677828 Free PMC article.
Cited by
-
Toxicological findings in suicides - frequency of antidepressant and antipsychotic substances.Forensic Sci Med Pathol. 2019 Mar;15(1):23-30. doi: 10.1007/s12024-018-0041-4. Epub 2018 Nov 5. Forensic Sci Med Pathol. 2019. PMID: 30397872
-
Denying renal transplantation to an adolescent medical cannabis user: An ethical case study.Pediatr Transplant. 2019 Aug;23(5):e13467. doi: 10.1111/petr.13467. Epub 2019 May 23. Pediatr Transplant. 2019. PMID: 31124250 Free PMC article.
-
Antidepressant prescription rates and suicide attempt rates from 2004 to 2016 in a nationally representative sample of adolescents in the USA.Epidemiol Psychiatr Sci. 2019 Oct;28(5):589-591. doi: 10.1017/S2045796018000136. Epub 2018 Apr 10. Epidemiol Psychiatr Sci. 2019. PMID: 29633680 Free PMC article. No abstract available.
-
Did the introduction and increased prescribing of antidepressants lead to changes in long-term trends of suicide rates?Eur J Public Health. 2021 Apr 24;31(2):291-297. doi: 10.1093/eurpub/ckaa204. Eur J Public Health. 2021. PMID: 33236104 Free PMC article.
-
Paroxetine-The Antidepressant from Hell? Probably Not, But Caution Required.Psychopharmacol Bull. 2016 Mar 1;46(1):77-104. Psychopharmacol Bull. 2016. PMID: 27738376 Free PMC article. Review.
References
-
- Healy D. Let them eat Prozac.New York University Press, 2004.
-
- Gøtzsche PC. Deadly psychiatry and organised denial.People’s Press, 2015.
-
- Whittington CJ, Kendall T, Fonagy P, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363: 1341-5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
