Functional Differences Between Placental Micro- and Macrovascular Endothelial Colony-Forming Cells
- PMID: 26819255
- PMCID: PMC4807658
- DOI: 10.5966/sctm.2014-0162
Functional Differences Between Placental Micro- and Macrovascular Endothelial Colony-Forming Cells
Abstract
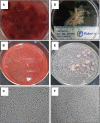
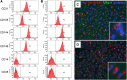
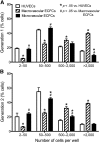
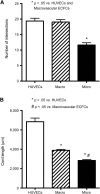
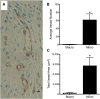
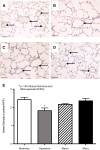
Alterations in the development of the placental vasculature can lead to pregnancy complications, such as preeclampsia. Currently, the cause of preeclampsia is unknown, and there are no specific prevention or treatment strategies. Further insight into the placental vasculature may aid in identifying causal factors. Endothelial colony-forming cells (ECFCs) are a subset of endothelial progenitor cells capable of self-renewal and de novo vessel formation in vitro. We hypothesized that ECFCs exist in the micro- and macrovasculature of the normal, term human placenta. Human placentas were collected from term pregnancies delivered by cesarean section (n = 16). Placental micro- and macrovasculature was collected from the maternal and fetal side of the placenta, respectively, and ECFCs were isolated and characterized. ECFCs were CD31(+), CD105(+), CD144(+), CD146(+), CD14(-), and CD45(-), took up 1,1'-dioctadecyl-3,3,3',3'-tetramethyl-indocarbocyanine perchlorate-labeled acetylated low-density lipoprotein, and bound Ulex europaeus agglutinin 1. In vitro, macrovascular ECFCs had a greater potential to generate high-proliferative colonies and formed more complex capillary-like networks on Matrigel compared with microvascular ECFCs. In contrast, in vivo assessment demonstrated that microvascular ECFCs had a greater potential to form vessels. Macrovascular ECFCs were of fetal origin, whereas microvascular ECFCs were of maternal origin. ECFCs exist in the micro- and macrovasculature of the normal, term human placenta. Although macrovascular ECFCs demonstrated greater vessel and colony-forming potency in vitro, this did not translate in vivo, where microvascular ECFCs exhibited a greater vessel-forming ability. These important findings contribute to the current understanding of normal placental vascular development and may aid in identifying factors involved in preeclampsia and other pregnancy complications.
Keywords: Angiogenesis; Endothelial progenitor cell; Placental vasculature; Preeclampsia; Stem cell.
©AlphaMed Press.
Figures
Similar articles
-
Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia.Hypertension. 2014 Jul;64(1):165-71. doi: 10.1161/HYPERTENSIONAHA.113.03058. Epub 2014 Apr 21. Hypertension. 2014. PMID: 24752434 Free PMC article.
-
Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta.Stem Cells Transl Med. 2013 Nov;2(11):839-47. doi: 10.5966/sctm.2013-0092. Epub 2013 Oct 8. Stem Cells Transl Med. 2013. PMID: 24106336 Free PMC article.
-
Adult venous endothelium is a niche for highly proliferative and vasculogenic endothelial colony-forming cells.J Vasc Surg. 2017 Dec;66(6):1854-1863. doi: 10.1016/j.jvs.2016.11.059. Epub 2017 Jun 24. J Vasc Surg. 2017. PMID: 28655551
-
Fetal endothelial colony-forming cells: Possible targets for prevention of the fetal origins of adult diseases.Placenta. 2024 Jan;145:80-88. doi: 10.1016/j.placenta.2023.12.006. Epub 2023 Dec 10. Placenta. 2024. PMID: 38100962 Review.
-
Endothelial progenitor cell subsets and preeclampsia: Findings and controversies.J Chin Med Assoc. 2017 Oct;80(10):615-622. doi: 10.1016/j.jcma.2017.06.013. Epub 2017 Jul 14. J Chin Med Assoc. 2017. PMID: 28716604 Review.
Cited by
-
Are all stem cells equal? Systematic review, evidence map, and meta-analyses of preclinical stem cell-based therapies for bronchopulmonary dysplasia.Stem Cells Transl Med. 2020 Feb;9(2):158-168. doi: 10.1002/sctm.19-0193. Epub 2019 Nov 20. Stem Cells Transl Med. 2020. PMID: 31746123 Free PMC article.
-
Current concepts on endothelial stem cells definition, location, and markers.Stem Cells Transl Med. 2021 Nov;10 Suppl 2(Suppl 2):S54-S61. doi: 10.1002/sctm.21-0022. Stem Cells Transl Med. 2021. PMID: 34724714 Free PMC article. Review.
-
Clonal isolation of endothelial colony-forming cells from early gestation chorionic villi of human placenta for fetal tissue regeneration.World J Stem Cells. 2020 Feb 26;12(2):123-138. doi: 10.4252/wjsc.v12.i2.123. World J Stem Cells. 2020. PMID: 32184937 Free PMC article.
-
Generation of vascular chimerism within donor organs.Sci Rep. 2021 Jun 28;11(1):13437. doi: 10.1038/s41598-021-92823-7. Sci Rep. 2021. PMID: 34183759 Free PMC article.
-
Unraveling the potential of endothelial progenitor cells as a treatment following ischemic stroke.Front Neurol. 2022 Sep 8;13:940682. doi: 10.3389/fneur.2022.940682. eCollection 2022. Front Neurol. 2022. PMID: 36158970 Free PMC article. Review.
References
-
- Kingdom J, Huppertz B, Seaward G, et al. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. - PubMed
-
- Demir R, Kayisli UA, Cayli S, et al. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27:535–539. - PubMed
-
- Ilekis JV, Reddy UM, Roberts JM. Preeclampsia—A pressing problem: An executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci. 2007;14:508–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

