The L, M, and S Segments of Rift Valley Fever Virus MP-12 Vaccine Independently Contribute to a Temperature-Sensitive Phenotype
- PMID: 26819307
- PMCID: PMC4794659
- DOI: 10.1128/JVI.02241-15
The L, M, and S Segments of Rift Valley Fever Virus MP-12 Vaccine Independently Contribute to a Temperature-Sensitive Phenotype
Abstract
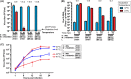
Rift Valley fever (RVF) is endemic to Africa, and the mosquito-borne disease is characterized by "abortion storms" in ruminants and by hemorrhagic fever, encephalitis, and blindness in humans. Rift Valley fever virus (RVFV; family Bunyaviridae, genus Phlebovirus) has a tripartite negative-stranded RNA genome (L, M, and S segments). A live-attenuated vaccine for RVF, the MP-12 vaccine, is conditionally licensed for veterinary use in the United States. MP-12 is fully attenuated by the combination of the partially attenuated L, M, and S segments. Temperature sensitivity (ts) limits viral replication at a restrictive temperature and may be involved with viral attenuation. In this study, we aimed to characterize the ts mutations for MP-12. The MP-12 vaccine showed restricted replication at 38°C and replication shutoff (100-fold or greater reduction in virus titer compared to that at 37°C) at 39°C in Vero and MRC-5 cells. Using rZH501 reassortants with either the MP-12 L, M, or S segment, we found that all three segments encode a temperature-sensitive phenotype. However, the ts phenotype of the S segment was weaker than that of the M or L segment. We identified Gn-Y259H, Gc-R1182G, L-V172A, and L-M1244I as major ts mutations for MP-12. The ts mutations in the L segment decreased viral RNA synthesis, while those in the M segment delayed progeny production from infected cells. We also found that a lack of NSs and/or 78kD/NSm protein expression minimally affected the ts phenotype. Our study revealed that MP-12 is a unique vaccine carrying ts mutations in the L, M, and S segments.
Importance: Rift Valley fever (RVF) is a mosquito-borne viral disease endemic to Africa, characterized by high rates of abortion in ruminants and severe diseases in humans. Vaccination is important to prevent the spread of disease, and a live-attenuated MP-12 vaccine is currently the only vaccine with a conditional license in the United States. This study determined the temperature sensitivity (ts) of MP-12 vaccine to understand virologic characteristics. Our study revealed that MP-12 vaccine contains ts mutations independently in the L, M, and S segments and that MP-12 displays a restrictive replication at 38°C.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Swanepoel R, Coetzer JAW. 2004. Rift Valley fever, p 1037–1070. In Coetzer JAW, Tustin RC (ed), Infectious diseases of livestock with special reference to southern Africa, 2nd ed Oxford University Press, Cape Town, South Africa.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

