Novel RNA-binding activity of MYF5 enhances Ccnd1/Cyclin D1 mRNA translation during myogenesis
- PMID: 26819411
- PMCID: PMC4797292
- DOI: 10.1093/nar/gkw023
Novel RNA-binding activity of MYF5 enhances Ccnd1/Cyclin D1 mRNA translation during myogenesis
Abstract
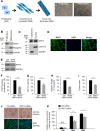
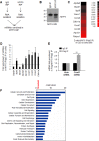
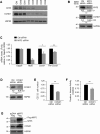
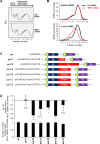
Skeletal muscle contains long multinucleated and contractile structures known as muscle fibers, which arise from the fusion of myoblasts into multinucleated myotubes during myogenesis. The myogenic regulatory factor (MRF) MYF5 is the earliest to be expressed during myogenesis and functions as a transcription factor in muscle progenitor cells (satellite cells) and myocytes. In mouse C2C12 myocytes, MYF5 is implicated in the initial steps of myoblast differentiation into myotubes. Here, using ribonucleoprotein immunoprecipitation (RIP) analysis, we discovered a novel function for MYF5 as an RNA-binding protein which associated with a subset of myoblast mRNAs. One prominent MYF5 target was Ccnd1 mRNA, which encodes the key cell cycle regulator CCND1 (Cyclin D1). Biotin-RNA pulldown, UV-crosslinking and gel shift experiments indicated that MYF5 was capable of binding the 3' untranslated region (UTR) and the coding region (CR) of Ccnd1 mRNA. Silencing MYF5 expression in proliferating myoblasts revealed that MYF5 promoted CCND1 translation and modestly increased transcription of Ccnd1 mRNA. Accordingly, overexpressing MYF5 in C2C12 cells upregulated CCND1 expression while silencing MYF5 reduced myoblast proliferation as well as differentiation of myoblasts into myotubes. Moreover, MYF5 silencing reduced myogenesis, while ectopically restoring CCND1 abundance partially rescued the decrease in myogenesis seen after MYF5 silencing. We propose that MYF5 enhances early myogenesis in part by coordinately elevating Ccnd1 transcription and Ccnd1 mRNA translation.
Published by Oxford University Press on behalf of Nucleic Acids Research 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
-
- Collins C.A., Olsen I., Zammit P.S., Heslop L., Petrie A., Partridge T.A., Morgan J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. - PubMed
-
- Moss F.P., Leblond C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971;170:421–435. - PubMed
-
- Chargé S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. - PubMed
-
- Hawke T.J., Garry D.J. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 2001;91:534–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

