Cyclic GMP-AMP Synthase Is Required for Cell Proliferation and Inflammatory Responses in Rheumatoid Arthritis Synoviocytes
- PMID: 26819496
- PMCID: PMC4706940
- DOI: 10.1155/2015/192329
Cyclic GMP-AMP Synthase Is Required for Cell Proliferation and Inflammatory Responses in Rheumatoid Arthritis Synoviocytes
Abstract
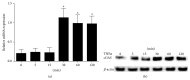
Rheumatoid arthritis (RA) is characterized by inflammatory cell infiltration, fibroblast-like synoviocytes (FLS) invasive proliferation, and joint destruction. Cyclic GMP-AMP synthase (cGAS) is a cytosolic DNA sensor that induces immune activation. In this study, we examined whether cGAS plays a role in RA FLS. In this study, cGAS was overexpressed in RA-FLS compared with OA FLS. TNFα stimulation induced cGAS expression in RA FLS. Overexpression of cGAS promoted the proliferation and knockdown of cGAS inhibited the proliferation of RA FLS. cGAS overexpression enhanced the production of proinflammatory cytokines and matrix metalloproteinases (MMPs) as well as AKT and ERK phosphorylation in TNFα-stimulated FLS. In contrast, cGAS silencing inhibited production of proinflammatory cytokines and matrix metalloproteinases (MMPs) as well as AKT and ERK phosphorylation in TNFα-stimulated FLS. These results suggest that cGAS activates the AKT and ERK pathways to promote the inflammatory response of RA FLS, and the development of strategies targeting cGAS may have therapeutic potential for human RA.
Figures




Similar articles
-
Sprouty2 suppresses the inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes through regulating the Raf/ERK and PTEN/AKT signals.Mol Immunol. 2015 Oct;67(2 Pt B):532-9. doi: 10.1016/j.molimm.2015.07.033. Epub 2015 Aug 8. Mol Immunol. 2015. PMID: 26265114
-
Accumulation of cytosolic dsDNA contributes to fibroblast-like synoviocytes-mediated rheumatoid arthritis synovial inflammation.Int Immunopharmacol. 2019 Nov;76:105791. doi: 10.1016/j.intimp.2019.105791. Epub 2019 Aug 28. Int Immunopharmacol. 2019. PMID: 31472320
-
Parallel comparison of fibroblast-like synoviocytes from the surgically removed hyperplastic synovial tissues of rheumatoid arthritis and osteoarthritis patients.BMC Musculoskelet Disord. 2019 Dec 7;20(1):591. doi: 10.1186/s12891-019-2977-2. BMC Musculoskelet Disord. 2019. PMID: 31812161 Free PMC article.
-
Metabolic Reprogramming in Stromal and Immune Cells in Rheumatoid Arthritis and Osteoarthritis: Therapeutic Possibilities.Eur J Immunol. 2025 Apr;55(4):e202451381. doi: 10.1002/eji.202451381. Eur J Immunol. 2025. PMID: 40170391 Free PMC article. Review.
-
Role of signaling lymphocytic activation molecule family of receptors in the pathogenesis of rheumatoid arthritis: insights and application.Front Pharmacol. 2023 Nov 6;14:1306584. doi: 10.3389/fphar.2023.1306584. eCollection 2023. Front Pharmacol. 2023. PMID: 38027031 Free PMC article. Review.
Cited by
-
Discovery and characterization of a novel cGAS covalent inhibitor for the treatment of inflammatory bowel disease.Acta Pharmacol Sin. 2023 Apr;44(4):791-800. doi: 10.1038/s41401-022-01002-5. Epub 2022 Oct 13. Acta Pharmacol Sin. 2023. PMID: 36229599 Free PMC article.
-
Beyond DNA sensing: expanding the role of cGAS/STING in immunity and diseases.Arch Pharm Res. 2023 Jun;46(6):500-534. doi: 10.1007/s12272-023-01452-3. Epub 2023 Jun 24. Arch Pharm Res. 2023. PMID: 37354378 Free PMC article. Review.
-
The cGAS-STING pathway: more than fighting against viruses and cancer.Cell Biosci. 2021 Dec 14;11(1):209. doi: 10.1186/s13578-021-00724-z. Cell Biosci. 2021. PMID: 34906241 Free PMC article. Review.
-
cGAS-STING pathway in oncogenesis and cancer therapeutics.Oncotarget. 2020 Jul 28;11(30):2930-2955. doi: 10.18632/oncotarget.27673. eCollection 2020 Jul 28. Oncotarget. 2020. PMID: 32774773 Free PMC article. Review.
-
The role of cGAS-STING signaling in rheumatoid arthritis: from pathogenesis to therapeutic targets.Front Immunol. 2024 Sep 25;15:1466023. doi: 10.3389/fimmu.2024.1466023. eCollection 2024. Front Immunol. 2024. PMID: 39386207 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

