Clinical Use of Tolerogenic Dendritic Cells-Harmonization Approach in European Collaborative Effort
- PMID: 26819498
- PMCID: PMC4706930
- DOI: 10.1155/2015/471719
Clinical Use of Tolerogenic Dendritic Cells-Harmonization Approach in European Collaborative Effort
Abstract
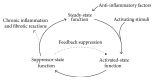
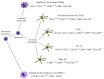
The number of patients with autoimmune diseases and severe allergies and recipients of transplants increases worldwide. Currently, these patients require lifelong administration of immunomodulatory drugs. Often, these drugs are expensive and show immediate or late-occurring severe side effects. Treatment would be greatly improved by targeting the cause of autoimmunity, that is, loss of tolerance to self-antigens. Accumulating knowledge on immune mechanisms has led to the development of tolerogenic dendritic cells (tolDC), with the specific objective to restrain unwanted immune reactions in the long term. The first clinical trials with tolDC have recently been conducted and more tolDC trials are underway. Although the safety trials have been encouraging, many questions relating to tolDC, for example, cell-manufacturing protocols, administration route, amount and frequency, or mechanism of action, remain to be answered. Aiming to join efforts in translating tolDC and other tolerogenic cellular products (e.g., Tregs and macrophages) to the clinic, a European COST (European Cooperation in Science and Technology) network has been initiated-A FACTT (action to focus and accelerate cell-based tolerance-inducing therapies). A FACTT aims to minimize overlap and maximize comparison of tolDC approaches through establishment of minimum information models and consensus monitoring parameters, ensuring that progress will be in an efficient, safe, and cost-effective way.
Figures

References
-
- Brent L., Brooks C. G., Medawar P. B., Simpson E. Transplantation tolerance. British Medical Bulletin. 1976;32(2):101–106. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation. 2009;9(supplement 3):S1–S155. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

