Rapid Point-of-Care Isothermal Amplification Assay for the Detection of Malaria without Nucleic Acid Purification
- PMID: 26819557
- PMCID: PMC4721682
- DOI: 10.4137/IDRT.S32162
Rapid Point-of-Care Isothermal Amplification Assay for the Detection of Malaria without Nucleic Acid Purification
Abstract
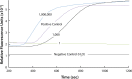
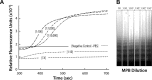
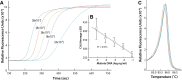
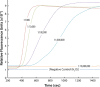
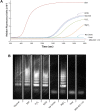
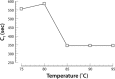
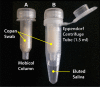
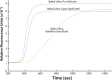
Malaria remains one of the most prevalent infectious diseases and results in significant mortality. Isothermal amplification (loop-mediated isothermal amplification) is used to detect malarial DNA at levels of ~1 parasite/µL blood in ≤30 minutes without the isolation of parasite nucleic acid from subject's blood or saliva. The technique targets the mitochondrial cytochrome oxidase subunit 1 gene and is capable of distinguishing Plasmodium falciparum from Plasmodium vivax. Malarial diagnosis by the gold standard microscopic examination of blood smears is generally carried out only after moderate-to-severe symptoms appear. Rapid diagnostic antigen tests are available but generally require infection levels in the range of 200-2,000 parasites/µL for a positive diagnosis and cannot distinguish if the disease has been cleared due to the persistence of circulating antigen. This study describes a rapid and simple molecular assay to detect malarial genes directly from whole blood or saliva without DNA isolation.
Keywords: Plasmodium falciparum; Plasmodium vivax; loop-mediated isothermal amplification (LAMP); malaria; molecular detection; point-of-care (POC).
Figures

References
-
- World Health Organization . World Malaria Report 2014. Geneva, Switzerland: World Health Organization; 2014.
-
- Snounou G, Renia L. The vaccine is dead—long live the vaccine. Trends Parasitol. 2007;23(4):129–132. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

