A New Highly Reactive and Low Lipophilicity Fluorine-18 Labeled Tetrazine Derivative for Pretargeted PET Imaging
- PMID: 26819667
- PMCID: PMC4716598
- DOI: 10.1021/acsmedchemlett.5b00330
A New Highly Reactive and Low Lipophilicity Fluorine-18 Labeled Tetrazine Derivative for Pretargeted PET Imaging
Abstract
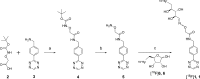
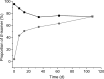
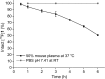
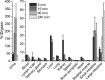
A new (18)F-labeled tetrazine derivative was developed aiming at optimal radiochemistry, fast reaction kinetics in inverse electron-demand Diels-Alder cycloaddition (IEDDA), and favorable pharmacokinetics for in vivo bioorthogonal chemistry. The radiolabeling of the tetrazine was achieved in high yield, purity, and specific activity under mild reaction conditions via conjugation with 5-[(18)F]fluoro-5-deoxyribose, providing a glycosylated tetrazine derivative with low lipophilicity. The (18)F-tetrazine showed fast reaction kinetics toward the most commonly used dienophiles in IEDDA reactions. It exhibited excellent chemical and enzymatic stability in mouse plasma and in phosphate-buffered saline (pH 7.41). Biodistribution in mice revealed favorable pharmacokinetics with major elimination via urinary excretion. The results indicate that the glycosylated (18)F-labeled tetrazine is an excellent candidate for in vivo bioorthogonal chemistry applications in pretargeted PET imaging approaches.
Keywords: Bioorthogonal chemistry; PET imaging; click chemistry; kinetics; tetrazine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Le Bars D. Fluorine-18 and medical imaging: Radiopharmaceuticals for positron emission tomography. J. Fluorine Chem. 2006, 127, 1488–1493. 10.1016/j.jfluchem.2006.09.015. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

