Potent Small Agonists of Protease Activated Receptor 2
- PMID: 26819675
- PMCID: PMC4716605
- DOI: 10.1021/acsmedchemlett.5b00429
Potent Small Agonists of Protease Activated Receptor 2
Abstract
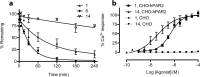
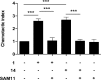
Many proteases cut the PAR2 N-terminus resulting in conformational changes that activate cells. Synthetic peptides corresponding to newly exposed N-terminal sequences of PAR2 also activate the receptor at micromolar concentrations. PAR2-selective small molecules reported here induce PAR2-mediated intracellular calcium signaling at nanomolar concentrations (EC50 = 15-100 nM, iCa(2+), CHO-hPAR2 cells). These are the most potent and efficient small molecule ligands to activate PAR2-mediated calcium release and chemotaxis, including for human breast and prostate cancer cells.
Keywords: Protease activated receptor 2; agonist; cyclohexylglycine; structure−activity relationships.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.P.F., L.L., M.K.Y., J.Y.S., and R.R. are named inventors on US Patent 8,927,503 and are co-authors on this manuscript. The patent is owned by the University of Queensland and concerns agonists and antagonists of PAR2.
Figures

Similar articles
-
Biased Signaling by Agonists of Protease Activated Receptor 2.ACS Chem Biol. 2017 May 19;12(5):1217-1226. doi: 10.1021/acschembio.6b01088. Epub 2017 Mar 14. ACS Chem Biol. 2017. PMID: 28169521
-
Mapping transmembrane residues of proteinase activated receptor 2 (PAR2) that influence ligand-modulated calcium signaling.Pharmacol Res. 2017 Mar;117:328-342. doi: 10.1016/j.phrs.2016.12.020. Epub 2016 Dec 16. Pharmacol Res. 2017. PMID: 27993717
-
Novel agonists and antagonists for human protease activated receptor 2.J Med Chem. 2010 Oct 28;53(20):7428-40. doi: 10.1021/jm100984y. J Med Chem. 2010. PMID: 20873792
-
Protease activated receptor 2 (PAR2) modulators: a patent review (2010-2015).Expert Opin Ther Pat. 2016;26(4):471-83. doi: 10.1517/13543776.2016.1154540. Epub 2016 Mar 3. Expert Opin Ther Pat. 2016. PMID: 26936077 Review.
-
Agonists and antagonists of protease activated receptors (PARs).Curr Med Chem. 2006;13(3):243-65. doi: 10.2174/092986706775476070. Curr Med Chem. 2006. PMID: 16475935 Review.
Cited by
-
Structural Characterization of Agonist Binding to Protease-Activated Receptor 2 through Mutagenesis and Computational Modeling.ACS Pharmacol Transl Sci. 2018 Oct 16;1(2):119-133. doi: 10.1021/acsptsci.8b00019. eCollection 2018 Nov 9. ACS Pharmacol Transl Sci. 2018. PMID: 32219208 Free PMC article.
-
The PAR2 inhibitor I-287 selectively targets Gαq and Gα12/13 signaling and has anti-inflammatory effects.Commun Biol. 2020 Nov 27;3(1):719. doi: 10.1038/s42003-020-01453-8. Commun Biol. 2020. PMID: 33247181 Free PMC article.
-
High Affinity Fluorescent Probe for Proteinase-Activated Receptor 2 (PAR2).ACS Med Chem Lett. 2019 Jun 6;10(7):1045-1050. doi: 10.1021/acsmedchemlett.9b00094. eCollection 2019 Jul 11. ACS Med Chem Lett. 2019. PMID: 31312406 Free PMC article.
-
Synthesis and initial pharmacology of dual-targeting ligands for putative complexes of integrin αVβ3 and PAR2.RSC Med Chem. 2020 Jul 9;11(8):940-949. doi: 10.1039/d0md00098a. eCollection 2020 Aug 1. RSC Med Chem. 2020. PMID: 33479689 Free PMC article.
-
Discovery of Novel Nonpeptidic PAR2 Ligands.ACS Med Chem Lett. 2020 May 22;11(6):1316-1323. doi: 10.1021/acsmedchemlett.0c00154. eCollection 2020 Jun 11. ACS Med Chem Lett. 2020. PMID: 32551018 Free PMC article.
References
-
- Zhao P.; Lieu T.; Barlow N.; Metcalf M.; Veldhuis N. A.; Jensen D. D.; Kocan M.; Sostegni S.; Haerteis S.; Baraznenok V.; Henderson I.; Lindstrom E.; Guerrero-Alba R.; Valdez-Morales E. E.; Liedtke W.; McIntyre P.; Vanner S. J.; Korbmacher C.; Bunnett N. W. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 2014, 289, 27215–27234. 10.1074/jbc.M114.599712. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

