Potent Small Agonists of Protease Activated Receptor 2
- PMID: 26819675
- PMCID: PMC4716605
- DOI: 10.1021/acsmedchemlett.5b00429
Potent Small Agonists of Protease Activated Receptor 2
Abstract
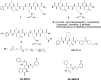
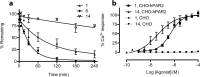
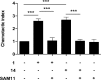
Many proteases cut the PAR2 N-terminus resulting in conformational changes that activate cells. Synthetic peptides corresponding to newly exposed N-terminal sequences of PAR2 also activate the receptor at micromolar concentrations. PAR2-selective small molecules reported here induce PAR2-mediated intracellular calcium signaling at nanomolar concentrations (EC50 = 15-100 nM, iCa(2+), CHO-hPAR2 cells). These are the most potent and efficient small molecule ligands to activate PAR2-mediated calcium release and chemotaxis, including for human breast and prostate cancer cells.
Keywords: Protease activated receptor 2; agonist; cyclohexylglycine; structure−activity relationships.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.P.F., L.L., M.K.Y., J.Y.S., and R.R. are named inventors on US Patent 8,927,503 and are co-authors on this manuscript. The patent is owned by the University of Queensland and concerns agonists and antagonists of PAR2.
Figures

References
-
- Zhao P.; Lieu T.; Barlow N.; Metcalf M.; Veldhuis N. A.; Jensen D. D.; Kocan M.; Sostegni S.; Haerteis S.; Baraznenok V.; Henderson I.; Lindstrom E.; Guerrero-Alba R.; Valdez-Morales E. E.; Liedtke W.; McIntyre P.; Vanner S. J.; Korbmacher C.; Bunnett N. W. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 2014, 289, 27215–27234. 10.1074/jbc.M114.599712. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

