Relationship between incident types and impact on patients in drug name errors: a correlational study
- PMID: 26819722
- PMCID: PMC4729157
- DOI: 10.1186/s40780-015-0011-x
Relationship between incident types and impact on patients in drug name errors: a correlational study
Abstract
Background: There are many reports regarding various medical institutions' attempts at incident prevention, but the relationship between incident types and impact on patients in drug name errors has not been studied. Therefore, we analyzed the relationship between them, while also assessing the relationship between preparation and inspection errors. Furthermore, the present study aimed to clarify the incident types that lead to severe patient damage.
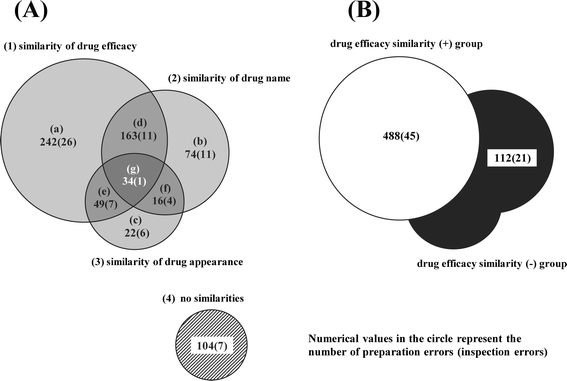
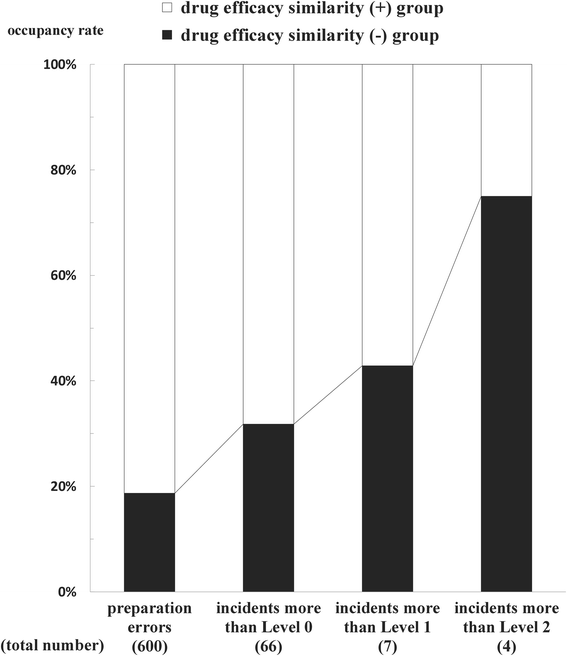
Methods: The investigation object in this study was restricted to "drug name errors", preparation and inspection errors in them were classified into three categories (similarity of drug efficacy, similarity of drug name, similarity of drug appearance) or two groups (drug efficacy similarity (+) group, drug efficacy similarity (-) group). Then, the relationship between preparation and inspection errors was investigated in three categories, the relationship between incident types and impact on patients was examined in two groups.
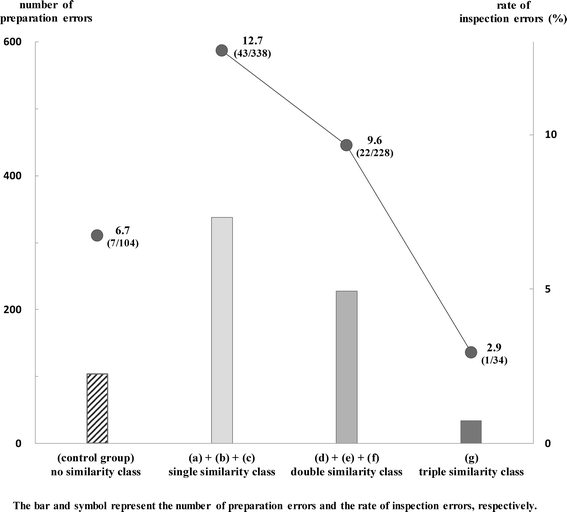
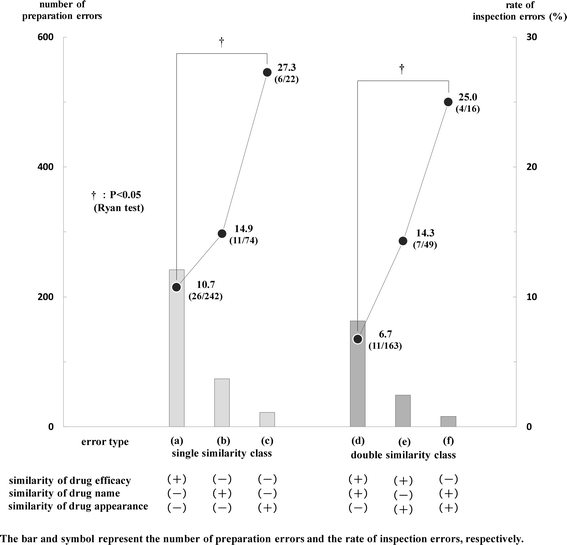
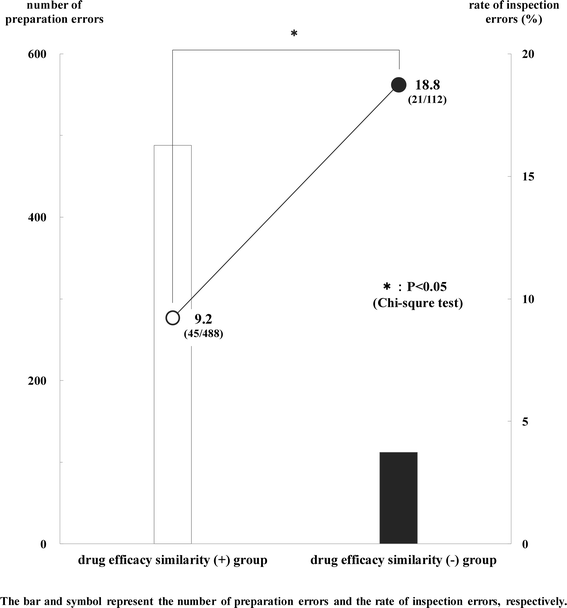
Results: The frequency of preparation errors was liable to be caused by the following order: similarity of drug efficacy > similarity of drug name > similarity of drug appearance. In contrast, the rate of inspection errors was liable to be caused by the following order: similarity of drug efficacy < similarity of drug name < similarity of drug appearance. In addition, the number of preparation errors in the drug efficacy similarity (-) group was fewer than that in the drug efficacy similarity (+) group. However, the rate of inspection errors in the drug efficacy similarity (-) group was significantly higher than that in the drug efficacy similarity (+) group. Furthermore, the occupancy rate of preparation errors, incidents more than Level 0, 1, and 2 in the drug efficacy similarity (-) group increased gradually according to the rise of patient damage.
Conclusions: Our results suggest that preparation errors caused by the similarity of drug appearance and/or drug name are likely to lead to the incidents (inspection errors), and these incidents are likely to cause severe damage to patients subsequently.
Keywords: Drug name errors; Impact on patients; Incidents; Inspection errors; Preparation errors.
Figures
References
-
- Watanabe H, Yoshida M, Nakahara A, Futagami S, Onoue R, Tsuji T, et al. Measures for Prevention of Dispensing Errors Based on ISO 9001 Certified Management System and Their Evaluation. Jap J Pharma Health Care Sci. 2006;32:824–34. doi: 10.5649/jjphcs.32.824. - DOI
-
- Tsuji T, Kakoki N, Irisa T, Kokubu C, Kanaya A, Hirakawa Y, et al. Estimation of risk ratio in classification of dispensing incident. Jap J Pharma Health Care Sci. 2013;39:528–35. doi: 10.5649/jjphcs.39.528. - DOI
-
- Tsuji T, Imai T, Kawashiri T, Kubota T, Hirakawa Y, Sueyasu M, et al. Effectiveness of ISO9001 Quality Management System for Preventing Dispensing Errors for Narcotic Drugs. Jap J Pharma Health Care Sci. 2012;38:350–8. doi: 10.5649/jjphcs.38.350. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

