Adherence to changing from brand-name to generic atorvastatin in newly treated patients: a retrospective cohort study using health insurance claims
- PMID: 26819723
- PMCID: PMC4728754
- DOI: 10.1186/s40780-015-0013-8
Adherence to changing from brand-name to generic atorvastatin in newly treated patients: a retrospective cohort study using health insurance claims
Abstract
Background: Effect of statin therapy has been reported to be associated with patient's adherence. Atorvastatin was available in Japan as a brand-name product beginning in 2000. The first atorvastatin generics were introduced in Japan in November 2011. The objective of this study was to analyze whether changing from a brand-name atorvastatin to a generic product would affect patient adherence.
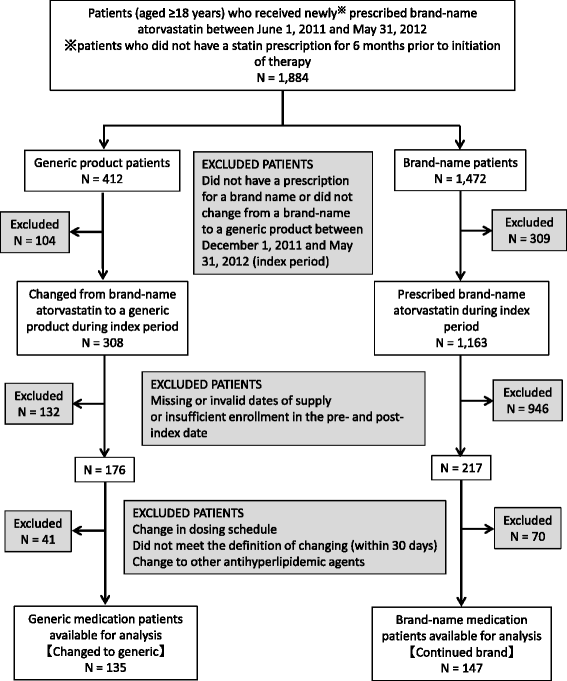
Methods: We conducted a retrospective cohort study that included adult patients who received newly prescribed brand-name atorvastatin between June 1, 2011 and May 31, 2012, using a health insurance claims database in Japan. Patients were classified by the presence or absence of changing to a generic during the 6 months from December 1, 2011 to May 31, 2012 (the index period). The first prescription date for the generic or brand product during the index period was defined as the index date. Adherence to therapy was assessed by the proportion of days covered (PDC) and persistence of treatment by time to discontinuation.
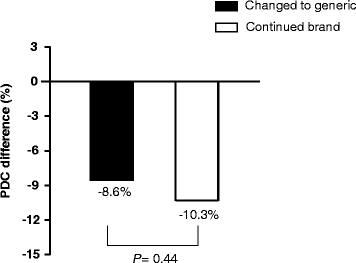
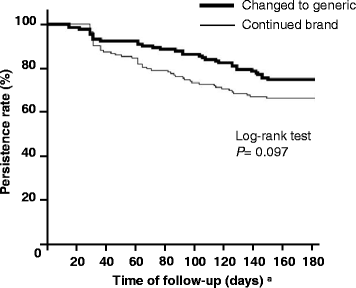
Results: There were 135 patients changing to generic atorvastatin and 147 continuing with the brand-name product. There was no significant difference in decrease of PDC from pre- to post-index date between the changed cohort and continued cohort (-8.6% vs -10.3%, respectively; P = 0.443). After adjusting for baseline covariates, including adherence in pre-index date, no statistically significant differences were observed in the adjusted odds of adherence between the cohorts (adjusted odds ratio = 0.83, 95% confidence interval (CI) = 0.46-1.53). There was also no significant difference in persistence between two cohorts in the 180-day after post-index date. After analysis of a Cox proportional hazard regression model controlling for baseline covariates, including adherence in pre-index date, no statistically significant differences were observed for the hazard of non-persistence between the cohorts (adjusted hazard ratio = 0.96, 95% CI = 0.60-1.53).
Conclusions: Changing from a brand-name atorvastatin to generic product did not affect adherence for patients newly treated with atorvastatin.
Keywords: Adherence; Atorvastatin; Claim database; Cohort study; Generic substitution; Persistence; Proportion of days covered.
Figures
Similar articles
-
Treatment persistence and adherence and their consequences on patient outcomes of generic versus brand-name statins routinely used to treat high cholesterol levels in Spain: a retrospective cost-consequences analysis.Lipids Health Dis. 2018 Dec 6;17(1):277. doi: 10.1186/s12944-018-0918-y. Lipids Health Dis. 2018. PMID: 30522491 Free PMC article.
-
Real-world Evidence for Adherence and Persistence with Atorvastatin Therapy.Cardiol Ther. 2021 Dec;10(2):445-464. doi: 10.1007/s40119-021-00240-8. Epub 2021 Sep 29. Cardiol Ther. 2021. PMID: 34586613 Free PMC article. Review.
-
Effect of Generic Competition on Atorvastatin Prescribing and Patients' Out-of-Pocket Spending.JAMA Intern Med. 2016 Sep 1;176(9):1317-23. doi: 10.1001/jamainternmed.2016.3384. JAMA Intern Med. 2016. PMID: 27367749
-
Comparison of adherence, persistence, and clinical outcome of generic and brand-name statin users: A retrospective cohort study using the Japanese claims database.J Cardiol. 2021 May;77(5):545-551. doi: 10.1016/j.jjcc.2020.12.003. Epub 2020 Dec 26. J Cardiol. 2021. PMID: 33371973
-
Physicians are more likely than non-physicians to use brand-name drugs to treat their chronic conditions.J Epidemiol Community Health. 2017 Sep;71(9):874-881. doi: 10.1136/jech-2016-208837. Epub 2017 Jul 3. J Epidemiol Community Health. 2017. PMID: 28674077 Review.
Cited by
-
Treatment persistence and adherence and their consequences on patient outcomes of generic versus brand-name statins routinely used to treat high cholesterol levels in Spain: a retrospective cost-consequences analysis.Lipids Health Dis. 2018 Dec 6;17(1):277. doi: 10.1186/s12944-018-0918-y. Lipids Health Dis. 2018. PMID: 30522491 Free PMC article.
-
Real-world Evidence for Adherence and Persistence with Atorvastatin Therapy.Cardiol Ther. 2021 Dec;10(2):445-464. doi: 10.1007/s40119-021-00240-8. Epub 2021 Sep 29. Cardiol Ther. 2021. PMID: 34586613 Free PMC article. Review.
-
Factors Influencing Drug Prescribing for Patients With Hospitalization History in Circulatory Disease-Patient Severity, Composite Adherence, and Physician-Patient Relationship: Retrospective Cohort Study.JMIR Aging. 2024 Dec 6;7:e59234. doi: 10.2196/59234. JMIR Aging. 2024. PMID: 39421979 Free PMC article.
-
Patients with Asthma Prescribed Once-Daily Fluticasone Furoate/Vilanterol or Twice-Daily Fluticasone Propionate/Salmeterol as Maintenance Treatment: Analysis from a Claims Database.Pulm Ther. 2018 Dec;4(2):135-147. doi: 10.1007/s41030-018-0084-4. Epub 2018 Oct 30. Pulm Ther. 2018. PMID: 32026395 Free PMC article.
-
Evaluation of pharmaceutical concerns in Germany: frequency and potential reasons.Pharm Pract (Granada). 2016 Jul-Sep;14(3):786. doi: 10.18549/PharmPract.2016.03.786. Epub 2016 Sep 15. Pharm Pract (Granada). 2016. PMID: 27785166 Free PMC article.
References
-
- Dunne S, Shannon B, Dunne C, Cullen W. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol. 2013;14:1. doi: 10.1186/2050-6511-14-1. - DOI - PMC - PubMed
-
- Generic Drug Saving In the U.S. (Sixth Annual Edition. 2014) [http://www.gphaonline.org/media/cms/GPhA_Savings_Report.9.10.14_FINAL.pdf]
LinkOut - more resources
Full Text Sources
Other Literature Sources

