Hyperglycemic adverse events following antipsychotic drug administration in spontaneous adverse event reports
- PMID: 26819726
- PMCID: PMC4728749
- DOI: 10.1186/s40780-015-0015-6
Hyperglycemic adverse events following antipsychotic drug administration in spontaneous adverse event reports
Abstract
Background: Antipsychotics are potent dopamine antagonists used to treat schizophrenia and bipolar disorder. The aim of this study was to evaluate the relationship between antipsychotic drugs and adverse hyperglycemic events using the FDA Adverse Event Reporting System (FAERS) database. In particular, we focused on adverse hyperglycemic events associated with atypical antipsychotic use, which are major concerns.
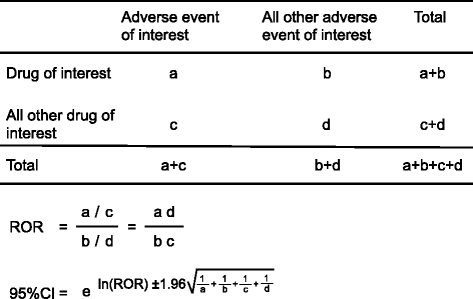
Findings: We analyzed reports of adverse hyperglycemic events associated with 26 antipsychotic drugs in the FAERS database from January 2004 to March 2013. The Standardized Medical Dictionary for Regulatory Activities Queries (SMQ) preferred terms (PTs) was used to identify adverse hyperglycemic events. The number of adverse hyperglycemic reports for the top eight antipsychotic drugs, quetiapine, olanzapine, risperidone, aripiprazole, haloperidol, clozapine, prochlorperazine, and chlorpromazine was 12,471 (28.9%), 8,423 (37.9%), 5,968 (27.0%), 4,045 (23.7%), 3,445 (31.5%), 2,614 (14.3%), 1,800 (19.8%), and 1,003 (35.7%), respectively. The reporting ratio increased with co-administration of multiple antipsychotic drugs. For example, adverse hyperglycemic events represented 21.6% of reports for quetiapine monotherapy, 39.9% for two-drug polypharmacy, and 66.3% for three-drug polypharmacy.
Conclusion: Antipsychotic drug polypharmacy may influence signal strength, and may be associated with hyperglycemia. After considering the causality restraints of the current analysis, further robust epidemiological studies are recommended.
Keywords: Adverse event reporting system; Antipsychotic drugs; Antipsychotic monotherapy; Antipsychotic polypharmacy; Hyperglycemic adverse events.
Figures
Similar articles
-
Characteristics of voluntary reporting of adverse drug events related to antipsychotics in Australia: 14-year analysis.Ther Adv Drug Saf. 2021 May 29;12:20420986211012854. doi: 10.1177/20420986211012854. eCollection 2021. Ther Adv Drug Saf. 2021. PMID: 34104400 Free PMC article.
-
Antipsychotics, glycemic disorders, and life-threatening diabetic events: a Bayesian data-mining analysis of the FDA adverse event reporting system (1968-2004).Ann Clin Psychiatry. 2008 Jan-Mar;20(1):21-31. doi: 10.1080/10401230701844612. Ann Clin Psychiatry. 2008. PMID: 18297583
-
Antipsychotics-associated serious adverse events in children: an analysis of the FAERS database.Int J Med Sci. 2015 Jan 5;12(2):135-40. doi: 10.7150/ijms.10453. eCollection 2015. Int J Med Sci. 2015. PMID: 25589889 Free PMC article.
-
A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation.Curr Med Chem. 2004 Feb;11(3):313-27. doi: 10.2174/0929867043456070. Curr Med Chem. 2004. PMID: 14965234 Review.
-
An open, large, 6-month naturalistic study of outcome in schizophrenic outpatients, treated with olanzapine.Hum Psychopharmacol. 2011 Jan;26(1):81-5. doi: 10.1002/hup.1173. Epub 2011 Feb 9. Hum Psychopharmacol. 2011. PMID: 23055416 Review.
Cited by
-
Descriptive analysis of reported adverse events associated with anti-obesity medications using FDA Adverse Event Reporting System (FAERS) databases 2013-2020.Int J Clin Pharm. 2022 Feb;44(1):172-179. doi: 10.1007/s11096-021-01330-2. Epub 2021 Sep 26. Int J Clin Pharm. 2022. PMID: 34564826
-
Pharmacovigilance study of anti-infective-related acute kidney injury using the Japanese adverse drug event report database.BMC Pharmacol Toxicol. 2021 Aug 30;22(1):47. doi: 10.1186/s40360-021-00513-x. BMC Pharmacol Toxicol. 2021. PMID: 34462002 Free PMC article.
-
Les médicaments qui interfèrent avec les bilans biologiques : revue de la littérature.Can J Hosp Pharm. 2021 Fall;74(4):378-385. doi: 10.4212/c-jhp.v74i4.3200. Can J Hosp Pharm. 2021. PMID: 34602626 Free PMC article. French.
-
Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese Adverse Drug Event Report database.J Pharm Health Care Sci. 2016 Jun 21;2:14. doi: 10.1186/s40780-016-0048-5. eCollection 2016. J Pharm Health Care Sci. 2016. PMID: 27330825 Free PMC article.
-
Adverse event profiles of solvent-based and nanoparticle albumin-bound paclitaxel formulations using the Food and Drug Administration Adverse Event Reporting System.SAGE Open Med. 2019 Mar 11;7:2050312119836011. doi: 10.1177/2050312119836011. eCollection 2019. SAGE Open Med. 2019. PMID: 30886713 Free PMC article.
References
-
- Haupt DW, Newcomer JW. Hyperglycemia and Antipsychotic medications. J Clin Psychiatry. 2001;62(Suppl 27):15–26. - PubMed
-
- Pharmaceuticals and Medical Devices Agency, Japan. Dear Healthcare Professional Letters. [http://www.pmda.go.jp/safety/info-services/drugs/calling-attention/esc-r...]
LinkOut - more resources
Full Text Sources
Other Literature Sources